Advertisements
Advertisements
Question
Draw a neat labelled diagram.
Electroplating
Solution
Electroplating-

APPEARS IN
RELATED QUESTIONS
What is an alloy?
Why do we apply paint on iron articles?
Explain the terms Corrosion
Two methods by which rusting of iron can be prevented are ______ and ______.
Explain why rusting of iron objects is faster in coastal areas than in deserts.
Choose the correct answer from the options given below:
Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as
A. Smelting
B. Ore-dressing
C. Calcination
D. Bessemerisation
What type of chemical reaction is involved in the corrosion of iron?
Name any three objects (or structures) which are gradually damaged by the corrosion of iron and steel.
Fill in the following blank with suitable word:
The process of depositing a thin layer of zinc on iron articles is called .............
What is the corrosion of iron known as?
Name two metals which resist corrosion due to the formation of a thin, hard and impervious layer of oxide on their surface.
What are the constituents of stainless steel?
Explain why, when a copper object remains in damp air for a considerable time, a green coating is formed on its surface. What is this process known as?
If copper is kept exposed to damp air for a considerable time, it gets a green coating on its surface. This is due to the formation of:
(a) hydrated copper sulphate
(b) copper oxide
(c) basic copper carbonate
(d) copper nitrate
Mention two uses of the following metals and non-metals
Iron
Name the metal which is a constituent of blood pigment?
What is corrosion? What are necessary conditions for corrosion?
Explain with reason:
Roasting is carried out on sulphide ores and not on carbonate ores.
Identify the process shown in the diagram and explain it in short
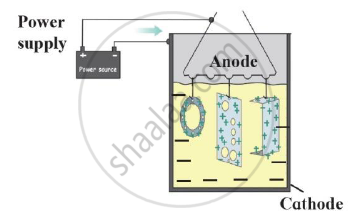
What are the adverse effects of corrosion?
Choose the correct alternative and rewrite the following:
Iron is _____________________.
Give reason.
Copper and brass utensils should be tinned.
Explain the term – rusting and give a word equation for the formation of rust. If polished iron nails are kept in three separate test tubes, state the contents in each test tube required, to prove the conditions for rusting.
State whether the statement given below is true or false. If false write the correct statement.
Either oxygen or moisture is essential for rusting.
_______ is an alloy made from iron, carbon and chromium.
Pressure cooker : Anodizing : : Silver plated spoons : _______
Find the odd one out and give its explanation.
Find the odd one out and give its explanation.
Write scientific reason.
On exposure to air, silver articles turn blackish after some time.
Write scientific reason.
Coins are made from metals and alloys.
Draw a neat labelled diagram.
Anodizing
What is rust?
The process of coating the surface of the metal with a thin layer of zinc is called ______
Give the equation for the formation of rust.
Copper objects lose their shine and form green coating of ____________.
The table shown below gives information about four substances: A, B, C and D.
| SUBSTANCE | MELTING POINT (K) | ELECTRICAL CONDUCTIVITY | |
| SOLID | LIQUID/ AQUEOUS | ||
| A | 295 | Good | Good |
| B | 1210 | Poor | Good |
| C | 1890 | Poor | Good |
| D | 1160 | Poor | Poor |
Identify Ionic compounds from the above given substances.
Gold plated ornaments is the example of ______.
| A process of forming a thick oxide of aluminium when aluminium is exposed to air. This coat makes it resistant to corrosion. Resistance can be improved by making a layer of oxide thinker. In this technique, the aluminium article is the anode, and the electrolyte is sulphuric acid. The anode reaction results in the formation of a black-coloured film of aluminium oxide on the anode. By putting appropriate dyes in the electrolytic solution, both coloured surface with the decorative finish is achieved. Kitchen articles like anodised such as pressure cookers, pans and frames of sliding windows are applications of this technique. |
- Name the anode and electrolyte used in this technique.
- How can we make aluminium articles made resistant to corrosion?
- Name the technique used to coat the aluminium articles.
In ______ process a layer of molten tin is deposited on metals.
