Advertisements
Advertisements
Question
Show that the average energy per molecule is proportional to the absolute temperature T of the gas.
Solution
The expression for the pressure exerted by a gas on the basis of kinetic theory of gas is given by
`P = 1/3(Nm)/v v_(rms)^2`
`PV=1/3xx2xx1/2xxNmv_(rms)^2`
`PV = 2/3E, ...(becauseE=1/2xxNmv_(rms)^2)`
By definition,
PV = RT ...(for one mole)
`RT = 2/3E`
`E=3/2RT`
`E=3/2Nk_BT ...(becauseR=Nk_B)`
`E/N=3/2k_BT`
`E/NmuT`
Average energy, per molecule, is directly proportional to absolute temperature T of the gas.
APPEARS IN
RELATED QUESTIONS
Can we define the temperature of (a) vacuum, (b) a single molecule?
A gas cylinder has walls that can bear a maximum pressure of 1.0 × 106 Pa. It contains a gas at 8.0 × 105 Pa and 300 K. The cylinder is steadily heated. Neglecting any change in the volume, calculate the temperature at which the cylinder will break.
The average translational kinetic energy of air molecules is 0.040 eV (1 eV = 1.6 × 10−19J). Calculate the temperature of the air. Boltzmann constant k = 1.38 × 10−23 J K−1.
An ideal gas is kept in a long cylindrical vessel fitted with a frictionless piston of cross-sectional area 10 cm2 and weight 1 kg in figure. The vessel itself is kept in a big chamber containing air at atmospheric pressure 100 kPa. The length of the gas column is 20 cm. If the chamber is now completely evacuated by an exhaust pump, what will be the length of the gas column? Assume the temperature to remain constant throughout the process.
Using figure, find the boiling point of methyl alcohol at 1 atm (760 mm of mercury) and at 0.5 atm.

If a = 0.72 and r = 0.24, then the value of tr is ______.
Answer in brief:
Compare the rms speed of hydrogen molecules at 127ºC with rms speed of oxygen molecules at 27ºC given that molecular masses of hydrogen and oxygen are 2 and 32 respectively.
If the density of oxygen is 1.44 kg/m3 at a pressure of 105 N/m2, find the root mean square velocity of oxygen molecules.
Calculate the ratio of the mean square speeds of molecules of a gas at 30 K and 120 K.
Earth’s mean temperature can be assumed to be 280 K. How will the curve of blackbody radiation look like for this temperature? Find out λmax. In which part of the electromagnetic spectrum, does this value lie? (Take Wien's constant b = 2.897 × 10−3 m K)
The power radiated by a perfect blackbody depends only on its ______
Above what temperature, all bodies radiate electromagnetic radiation?
What is the microscopic origin of temperature?
Explain in detail the kinetic interpretation of temperature.
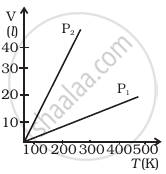
Volume versus temperature graphs for a given mass of an ideal gas are shown in figure at two different values of constant pressure. What can be inferred about relation between P1 and P2?
The molecules of a given mass of a gas have root mean square speeds of 100 ms−1 at 27°C and 1.00 atmospheric pressure. What will be the root mean square speeds of the molecules of the gas at 127°C and 2.0 atmospheric pressure?
An insulated container containing monoatomic gas of molar mass m is moving with a velocity vo. If the container is suddenly stopped, find the change in temperature.
A proton, a deuteron and an α-particle with same kinetic energy enter into a uniform magnetic field at right angle to magnetic field. The ratio of the radii of their respective circular paths is ______.
Two gases A and B are at absolute temperatures of 360 K and 420 K, respectively. The ratio of the average kinetic energy of the molecules of gas B to that of gas A is ______.
When a particle oscillates simple harmonically, its kinetic energy varies periodically. If frequency of the particle is n, then the frequency of the kinetic energy is ______.
If a = 0. 72 and t = 0.04, then the value of r is ______.
At what temperature will therms velocity of a gas be four times its value at STP?
2000 calories of radiant heat is incident on a body. If the body absorbs 550 calories of heat, find the coefficient of emmission of the body.
