Advertisements
Advertisements
Question
Using electron-dot diagrams which show only the outermost shell electrons, show how a molecule of oxygen, O2, is formed from two oxygen atoms. What name is given to this type of bonding? (At. No. of oxygen = 8)
Solution
The atomic number of oxygen is 8. Its electronic configuration is 2, 6. Hence it has six electrons in its outermost shell.
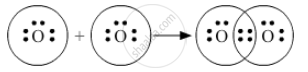
In an oxygen molecule, one oxygen atom shares its two electrons with another oxygen atom. Hence this type of bonding is called covalent bonding.
APPEARS IN
RELATED QUESTIONS
Write structural formula of Methane.
Write the number of covalent bonds in the molecule of butane, C4H10.
Butanone is a four-carbon compound with the functional group:
Buckminsterfullerene is spherical molecule in which 60 carbon atoms are arranged in interlocking hexagonal and pentagonal rings of carbon atoms.
How many pentagons of carbon atoms are present in one molecule of buckminsterfullerene?
Fill in the blank in the following sentence:
Two chlorine atoms combine to form a molecule. The bond between them is known as ..........
Give the formula of the compound that would be formed by the combination of the following pair of elements:
K and H
Draw the electron-dot structure of NH3 and state the type of bonding.
What is graphite?
State any two uses of graphite.
Write answer as directed.
What causes the existance of very large number of carbon compound ?
The molecule which contains a triple covalent bond is ______.
State the type of bonding in the following molecule.
Water
Draw the electron dot diagram and structure of magnesium chloride.
Draw the electron dot structure of covalent compound methane (non polar) and HCL (polar) and give two difference between them.
Write an Explanation.
Alkane
Write scientific reason.
Benzene compounds are called aromatic compounds.
Explain covalent bond with example.
Which of the following compounds of carbon does not consist of ions?
Name the following:
\[\ce{CH3 - CH2CH = CH2}\]
In covalent compounds, the bond is formed due to ______ of electrons.
