English Medium
Academic Year: 2022-2023
Date & Time: 4th March 2023, 10:30 am
Duration: 3h
Advertisements
General Instructions:
- This question paper consists of 39 questions in 5 sections.
- All questions are compulsory. However, an internal choice is provided in some questions. Students are expected to attempt only one of these questions.
- Section A consists of 20 objective type questions carrying 1 mark each. Q. No. 17 to 20 are Assertion - Reasoning based questions.
- Section B consists of 6 Very Short questions carrying 02 marks each. Answers to these questions should be in the range of 30 to 50 words.
- Section C consists of 7 Short Answer type questions carrying 03 marks each. Answers to these questions should be in the range of 50 to 80 words
- Section D consists of 3 Long Answer type questions carrying 05 marks each. Answers to these questions should be in the range of 80 to 120 words.
- Section E consists of 3 source-based/case-based units of assessment of 04 marks each with sub-parts.
- There is no overall choice. However, an internal choice has been provided in some Sections.
When sodium bicarbonate reacts with dilute hydrochloric acid, the gas evolved is ______.
Hydrogen; it given pop sound with burning match stick.
Hydrogen; it turns lime water milky.
Carbon dioxide; it turns lime water milky.
Carbon dioxide; it blows off a burning match stick with a pop sound.
Chapter:
When aqueous solutions of potassium iodide and lead nitrate are mixed an insoluble substance separates out. The chemical equation for the reaction involved is:
\[\ce{KI + PbNO_3 ->PbI + KNO3}\]
\[\ce{2KI + Pb(NO_3)2 ->PbI2 + 2KNO3}\]
\[\ce{KI + Pb(NO_3)2 ->PbI + KNO3}\]
\[\ce{KI + Pb(NO_3)2 ->PbI2 + 2KNO3}\]
Chapter: [0.01] Chemical Reactions and Equations
A metal ribbon 'X' bums in oxygen with a dazzling white flame forming a white ash 'Y'. The correct description of X, Y and the type of reaction is:
X = Ca; Y = CaO;
Type of reaction = Decomposition
X = Mg; Y = MgO;
Type of reaction = Combination
X = Al; Y = Al2O3;
Type of reaction = Thermal decomposition
X = Zn; Y = ZnO;
Type of reaction = Endothermic
Chapter: [0.01] Chemical Reactions and Equations
Acid present in tomato is ______.
Methanoic acid
Acetic acid
Lactic acid
Oxalic acid
Chapter: [0.02] Acids, Bases and Salts
Sodium hydroxide is termed an alkali while Ferric hydroxide is not because ______.
Sodium hydroxide is a strong base, while Ferric hydroxide is a weak base.
Sodium hydroxide is a base which is soluble in water while Ferric hydroxide is also a base but it is not soluble in water.
Sodium hydroxide is a strong base while Ferric hydroxide is a strong acid.
Sodium hydroxide and Ferric hydroxide both are strong base but the solubility of sodium hydroxide in water is comparatively higher than that of Ferric hydroxide.
Chapter: [0.02] Acids, Bases and Salts
The name of the salt used to remove permanent hardness of water is ______.
Sodium hydrogen carbonate (NaHCO3)
Sodium chloride (NaCl)
Sodium carbonate decahydrate (Na2CO3.10H2O)
Calcium sulphate hemihydrate (CaSO4.`1/2`H2O)
Chapter: [0.02] Acids, Bases and Salts
The electron dot structure of chlorine molecule is:

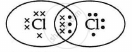
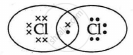

Chapter: [0.03] Metals and Non Metals [0.04] Carbon and its Compounds
Observe the following diagram and identify the process and its significance from the following options:

Evaporation: maintains water contents in leaf cells.
Transpiration: creates a suction force which pulls water inside the plant.
Excretion: helps in excreting out waste water from the plant.
Translocation: helps in transporting materials from one cell to another.
Chapter: [0.05] Life Processes
Water in the root enters due to ______.
The function of the root to absorb water.
difference in the concentration of ions between the root and the soil.
excess water present in the soil.
diffusion of water in the roots.
Chapter: [0.05] Life Processes
Which one of the given statements in incorrect:
DNA has the complete information for a particular characteristic.
DNA is the molecule responsible for the inheritance of characters from parents to offsprings.
Change in information will produce a different protein.
Characteristics will remain the same even if protein changes.
Chapter: [0.08] Heredity
Sensory nerve of a reflex arc carries information from the receptor cells to the ______.
spinal cord
brain
muscles of the effector organ
bones of the receptor organ
Chapter: [0.06] Control and Co-ordination
The number of chromosomes in parents and offsprings of a particular species undergoing sexual reproduction remain constant due to:
doubling of chromosomes after zygote formation.
the halving of chromosomes during gamete formation.
doubling of chromosomes after gamete formation.
the halving of chromosomes after gamete formation.
Chapter: [0.07] How do Organisms Reproduce?
Two LED bulbs of 12W and 6W are connected in series. If the current through 12W bulb is 0.06A the current through 6W bulb will be ______.
0.04 A
0.06 A
0.08 A
0.12 A
Chapter: [0.12] Magnetic Effects of Electric Current
The correct pattern of magnetic field lines of the field produced by a current carrying circular loop is:



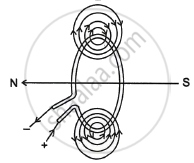
Chapter: [0.12] Magnetic Effects of Electric Current
The resistance of a resistor is reduced to half of its initial value. If other parameters of the electrical circuit remain unaltered, the amount of heat produced in the resistor will become ______.
four times
two times
half
one forth
Chapter: [0.11] Electricity
An alpha particle enters a uniform magnetic field as shown. The direction of force experienced by the alpha particle is ______.
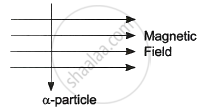
towards right
into the page
towards left
out of the page
Chapter: [0.12] Magnetic Effects of Electric Current
Assertion (A): Reaction of Quicklime with water is an exothermic reaction.
Reason (R): Quicklime reacts vigorously with water releasing a large amount of heat.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter: [0.02] Acids, Bases and Salts
Assertion (A): In humans, if gene (B) is responsible for black eyes and gene (b) responsible for brown eyes, then the colour of eyes of the progeny having gene combination Bb, bb or BB will be black only.
Reason (R): The black colour of the eyes is a dominant trait.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter: [0.08] Heredity
Assertion (A): The inner walls of the small intestine have finger like projections called villi which are rich in blood.
Reason (R): These villi have large surface area to help the small intestine in completing the digestion of food.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter: [0.05] Life Processes
Assertion (A): A current carrying straight conductor experiences a force when placed perpendicular to the direction of magnetic field.
Reason (R): The net charge on a current carrying conductor is always zero.
Both (A) and (R) are true and (R) is the correct explanation of (A).
Both (A) and (R) are true but (R) is not the correct explanation of (A).
(A) is true, but (R) is false.
(A) is false, but (R) is true.
Chapter: [0.12] Magnetic Effects of Electric Current
Name a plant hormone responsible for bending of a shoot of a plant when it is exposed to unidirectional light. How does is promote phototropism?
Chapter: [0.06] Control and Co-ordination
Name the part of brain which is responsible for following actions:
- Maintaining posture and balance
- Beating of heart
- Thinking
- Blood pressure
Chapter: [0.06] Control and Co-ordination [0.06] Control and Co-ordination
Where are auxins synthesized in a plant? Which organ of the plant shows:
- Positive phototropism
- Negative geotropism
- Positive hydrotropism
Chapter: [0.06] Control and Co-ordination
Write one specific function each of the following organs in relation with excretion in human beings:
- Renal Artery
- Urethra
- Glomerulus
- Tubular part of nephron
Chapter: [0.05] Life Processes
What is the other name of 'tissue fluid'? Write its two functions.
Chapter: [0.05] Life Processes
"Although gardens are created by man but they are considered to be an ecosystem." Justify this statement.
Chapter: [0.13] Our Environment
Use of several pesticides which results in excessive accumulation of pesticides in rivers of ponds, is a matter of deep concern. Justify this statement.
Chapter: [0.13] Our Environment
Advertisements
Consider the following salt:
YCl
What would be the pH of the solution if, in YCl, Y is sodium? Give a reason for your answer.
Chapter: [0.02] Acids, Bases and Salts
Consider the following salt:
NH4X
If in salt NH4X, X is nitrate, then its solution will give what colour with a universal indicator? Why?
Chapter: [0.02] Acids, Bases and Salts
Consider the following salt:
\[\ce{ZCO3}\]
What would be the change in colour in blue litmus if \[\ce{ZCO3}\] is added to it and Z is potassium?
Chapter: [0.02] Acids, Bases and Salts
Suggest a safe procedure of diluting a strong concentrated acid.
Chapter: [0.02] Acids, Bases and Salts
Name the salt formed when sulphuric acid is added to sodium hydroxide and write its pH.
Chapter: [0.02] Acids, Bases and Salts
Dry HCl gas does not change the colour of dry blue litmus paper. Why?
Chapter: [0.02] Acids, Bases and Salts
The magnification produced when an object is placed at a distance of 20 cm from a spherical mirror is +1/2. Where should the object be placed to reduce the magnification to +1/3.
Chapter: [0.09] Light - Reflection and Refraction
Define the following terms in the context of a diverging mirror:
- Principal focus
- Focal length
Draw a labelled ray diagram to illustrate your answer.
Chapter: [0.09] Light - Reflection and Refraction
An object of height 10 cm is placed 25 cm away from the optical centre of a converging lens of focal length 15 cm. Calculate the image distance and height of the image formed.
Chapter: [0.09] Light - Reflection and Refraction
The power of a lens is +4D. Find the focal length of this lens. An object is placed at a distance of 50 cm from the optical centre of this lens. State the nature and magnification of the image formed by the lens and also draw a ray diagram to justify your answer.
Chapter: [0.09] Light - Reflection and Refraction
Why is an alternating current (A.C.) considered to be advantageous over direct current (D.C.) for the long distance transmission of electric power?
Chapter: [0.12] Magnetic Effects of Electric Current
How is the type of current used in household supply different from the one given by a battery of dry cells?
Chapter: [0.12] Magnetic Effects of Electric Current
How does an electric fuse prevent the electric circuit and the appliances from a possible damage due to short circuiting or overloading.
Chapter: [0.12] Magnetic Effects of Electric Current
For the current carrying solenoid as shown, draw magnetic field lines and give reason to explain that out of the three points A, B and C, at which point the field strength is maximum and at which point it is minimum?

Chapter: [0.12] Magnetic Effects of Electric Current
Define the term dispersion of white light.
Chapter: [0.1] The Human Eye and the Colourful World
State the colour which bends:
- The most.
- The least while passing through a glass prism.
Chapter: [0.1] The Human Eye and the Colourful World
Draw a ray diagram to show the dispersion of white light.
Chapter: [0.1] The Human Eye and the Colourful World
What is a rainbow? Draw a labelled diagram to show the formation of a rainbow.
Chapter: [0.1] The Human Eye and the Colourful World
Draw the structure of butanoic acid.
Chapter: [0.04] Carbon and its Compounds
Draw the structure of the following compound:
Chloropentane
Chapter:
How are structure (i) and structure (ii) given below related to one another? Give reason to justify your answer.
Structure (i) \[\begin{array}{cc} \ce{CH3}\phantom{............}\ce{CH3}\\ \backslash\phantom{..........}/\\ \ce{CH - CH}\\ /\phantom{..........}\backslash\\ \ce{CH3}\phantom{............}\ce{CH3} \end{array}\]
Structure (ii) \[\begin{array}{cc} \ce{CH3}\phantom{...........}\\ \backslash\phantom{.......}\\ \ce{CH3 - C - CH2CH3}\\ /\phantom{.......}\\ \ce{CH3}\phantom{...........} \end{array}\]
Chapter:
Advertisements
Differentiate between saturated and unsaturated carbon compounds on the basis of their general formula.
Chapter:
What happens when a small piece of sodium is dropped in ethanol? Write the equation for this reactions.
Chapter: [0.04] Carbon and its Compounds
Why is glacial acetic acid called so?
Chapter: [0.04] Carbon and its Compounds
What happens when ethanol is heated at 443K in the presence of conc. H2SO4? Write the role of conc. H2SO4 in this case.
Chapter: [0.04] Carbon and its Compounds
Write an equation showing saponification.
Chapter: [0.04] Carbon and its Compounds
Name and explain the two modes of asexual reproduction observed in hydra.
Chapter: [0.07] How do Organisms Reproduce?
What is vegetative propagation?
Chapter: [0.07] How do Organisms Reproduce?
List two advantages of vegetative propagation technique.
Chapter: [0.07] How do Organisms Reproduce?
Write the chemical equation for the following:
Combustion of methane
Chapter: [0.04] Carbon and its Compounds
Write the chemical equation for the following:
Oxidation of ethanol
Chapter: [0.04] Carbon and its Compounds
Write the chemical equation for the following:
Hydrogenation of ethene
Chapter: [0.04] Carbon and its Compounds
Write the chemical equation for the following:
Esterification Reaction
Chapter: [0.04] Carbon and its Compounds
Write the chemical equation for the following:
Saponification Reaction
Chapter: [0.04] Carbon and its Compounds
Draw two structural isomers of butane.
Chapter: [0.04] Carbon and its Compounds
Draw the structure of propanol.
Chapter: [0.04] Carbon and its Compounds
Draw the structure of propanone.
Chapter: [0.04] Carbon and its Compounds
Name the third homologue of alcohols.
Chapter: [0.04] Carbon and its Compounds
Name the third homologue of aldehydes.
Chapter: [0.04] Carbon and its Compounds
Name the following:
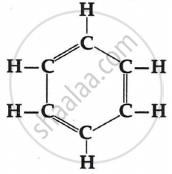
Chapter: [0.04] Carbon and its Compounds
Name the following:
\[\ce{CH3 - CH2CH = CH2}\]
Chapter: [0.03] Metals and Non Metals [0.04] Carbon and its Compounds
Show the covalent bond formation in nitrogen molecule.
Chapter: [0.03] Metals and Non Metals [0.04] Carbon and its Compounds
The melting points and boiling points of some ionic compounds are given below:
| Compound | Melting Point (K) |
Boiling Point (K) |
| NaCl |
1074 |
1686 |
| LiCl | 887 | 1600 |
| CaCl2 | 1045 | 1900 |
| CaO | 2850 | 3120 |
| MgCl2 | 981 | 1685 |
These compounds are termed ionic because they are formed by the transfer of electrons from a metal to a non-metal. The electron transfer in such compounds is controlled by the electronic configuration of the elements involved. Every element tends to attain a completely filled valence shell of its nearest noble gas or a stable octet.
- Show the electron transfer in the formation of magnesium chloride.
- List two properties of ionic compounds other than their high melting and boiling points.
- (A) While forming an ionic compound say sodium chloride how does sodium atom attain its stable configuration?
OR
(B) Give reasons:
(i) Why do ionic compounds in the solid state not conduct electricity?
(ii) What happens at the cathode when electricity is passed through an aqueous solution of sodium chloride?
Chapter:
| The most obvious outcome of the reproduction process is the generation of individuals of similar design, but in sexual reproduction they may not be exactly alike. The resemblances as well as differences are marked. The rules of heredity determine the process by which traits and characteristics are reliably inherited. Many experiments have been done to study the rules of inheritance. |
- Why an offspring of human being is not a true copy of his parents in sexual reproduction?
- While performing experiments on inheritance in plants, what is the difference between F1 and F2 generation?
- (A) Why do we say that variations are useful for the survival of a species over time?
OR
(B) Study Mendel's cross between two plants with a pair of contrasting characters.
RRYY × rryy Round Yellow Wrinkled Green He observed 4 types of combinations in F2 generation. Which of these were new combinations? Why do new features which are not present in the parents, appear in F2 generation?
Chapter: [0.07] How do Organisms Reproduce?
| The ability of medium to refract light is expressed in terms of its optical density. Optical density has a definite connotation. It is not the same as mass density. On comparing two media, the one with the large refractive index is optically denser medium than the other. The other medium with a lower refractive index is optically rarer. Also the speed of light through a given medium is inversely proportional to its optical density. |
- Determine the speed of light in diamond if the refractive index of diamond with respect to vacuum is 2.42. Speed of light in vacuum is 3 × 108 m/s.
- Refractive indices of glass, water and carbon disulphide are 1.5, 1.33 and 1.62 respectively. If a ray of light is incident in these media at the same angle (say θ), then write the increasing order of the angle of refraction in these media.
- (A) The speed of light in glass is 2 × 108 m/s and is water is 2.25 × 108 m/s.
(a) Which one of the two optically denser and why?
(b) A ray of light is incident normally at the water glass interface when it enters a thick glass container filled with water. What will happen to the path of the ray after entering the glass? Give reason.
OR
(B) The absolute refractive indices of glass and water are 4/3 and 3/2, respectively. If the speed of light in glass is 2 × 108 m/s, calculate the speed of light in (i) vacuum (ii) water.
Chapter: [0.09] Light - Reflection and Refraction
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 10 Science with solutions 2022 - 2023
Previous year Question paper for CBSE Class 10 Science-2023 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 10.
How CBSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Science will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
