Advertisements
Online Mock Tests
Chapters
2: Solutions
3: Ionic Equilibria
4: Chemical Thermodynamics
5: Electrochemistry
6: Chemical Kinetics
7: Elements of Groups 16, 17 and 18
8: Transition and Inner transition Elements
9: Coordination Compounds
▶ 10: Halogen Derivatives
11: Alcohols, Phenols and Ethers
12: Aldehydes, Ketones and Carboxylic acids
13: Amines
14: Biomolecules
15: Introduction to Polymer Chemistry
16: Green Chemistry and Nanochemistry
![SCERT Maharashtra solutions for Chemistry [English] 12 Standard HSC chapter 10 - Halogen Derivatives SCERT Maharashtra solutions for Chemistry [English] 12 Standard HSC chapter 10 - Halogen Derivatives - Shaalaa.com](/images/chemistry-english-12-standard-hsc_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 10: Halogen Derivatives
Below listed, you can find solutions for Chapter 10 of Maharashtra State Board SCERT Maharashtra for Chemistry [English] 12 Standard HSC.
SCERT Maharashtra solutions for Chemistry [English] 12 Standard HSC 10 Halogen Derivatives Multiple choice questions
1 Mark
The type of mono halogen derivative in which a halogen atom is bonded to sp3 hybridized carbon atom next to carbon-carbon double bond is ______________
alkyl halide
allylic halide
vinylic halide
benzylic halide
Aromatic electrophilic substitution with iodine can be carried out using _____________
HNO3
HCl
HI
H3PO4
For the isomeric dihalobenzenes, melting point of __________
ortho isomer is higher
meta isomer is higher
para isomer is higher
all isomers is nearly same
Optical activity of a molecule is associated with _______________
plane polarized light
3-D structure of a molecule
achiral molecule
superimposable mirror images
Propane nitrile can be prepared by heating ____________
ethyl bromide with alcoholic KCN
propyl bromide with alcoholic KCN
ethyl bromide with alcoholic AgCN
propyl bromide with alcoholic AgCN
The following will react faster by SN1 mechanism
1-chloropropane
2-chloropropane
2-chloro-2-methylpropane
chloroethane
Major product of the following reaction is _______________
\[\ce{CH3-CH2-Mg-Br + NH3 ->?}\]
CH3-CH2-Mg-NH2
CH3-CH3
Mg-Br-NH2
CH3-CH2-Br
SCERT Maharashtra solutions for Chemistry [English] 12 Standard HSC 10 Halogen Derivatives Very Short Answer Questions
1 Mark
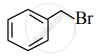
Write the IUPAC name of the following.
Write the major product of the following reaction.
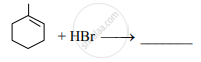
Write the correct decreasing order of boiling point for bromomethane, chloroform, dibromomethane and bromoform.
Write IUPAC name of the product ‘B’ in the following reaction sequence.
\[\ce{C2H5OH ->[NaBr/H2SO4][\Delta] A ->[Nal][acetone]B}\]
Nucleophilic substitution reaction of 2, 4-dinitrochlorobenzene is faster than p-nitrochlorobenzene. Give reason.
Name the reagent used to convert alkyl halide to ester.
Write the correct order of increasing ease of dehydrohalogenation.
\[\ce{\underset{\text{(I)}}{CH3 - CH2 - CH2 - Cl}}\]
\[\ce{\underset{\text{(II)}}{CH3 - CH(Cl) - CH3}}\]
\[\begin{array}{cc}
\ce{CH3}\\
|\\
\ce{CH3-C-Cl}\phantom{...}\\
|\\
\ce{CH3}\\
\ce{(III)}
\end{array}\]
SCERT Maharashtra solutions for Chemistry [English] 12 Standard HSC 10 Halogen Derivatives Short Answer Questions (Type-Ⅰ)
2 Marks
Explain. Aryl halides are less reactive than alkyl halides towards nucleophilic substitution reactions.
Explain reactions of haloarenes with sodium metal.
Give reason. Though alkyl halides are moderately polar, they are insoluble in water.
Explain optical activity of 2-chlorobutane
Distinguish between SN1 and SN2 mechanism of substitution reaction.
Explain primary benzylic halide shows higher reactivity by SN1 mechanism than other primary alkyl halide.
SCERT Maharashtra solutions for Chemistry [English] 12 Standard HSC 10 Halogen Derivatives Short Answer Questions (Type-II)
3 Marks
Explain the factors affecting SN1 and SN2 mechanism.
Explain aqueous alkaline hydrolysis of tert. butyl bromide.
How are the following conversions carried out?
propene to 1-iodopropane
How are the following conversions carried out?
propene to 2-nitropropane
How are the following conversions carried out?
benzene to biphenyl
What is Grignard reagent? How is it prepared? Why are they prepared under anhydrous condition?
Write chemical equations indicating the action of following on bromobenzene.
CH3COCl/anhy. AlCl3
Write chemical equations indicating the action of following on bromobenzene.
fuming H2SO4
Write chemical equations indicating the action of following on bromobenzene.
conc. HNO3/conc. H2SO4
An organic compound A with molecular formula C4H10O on treatment with phosphorus pentachloride gives alkyl chloride. Alkyl chloride on treatment with Mg in presence of dry ether gives a highly reactive compound B.
Compound B reacts with water to give hydrocarbon C. Alkyl chloride on treatment with Na in dry ether as a solvent gives alkane, 2,2,3,3-tetramethylbutane. Identify ‘A’, ‘B’, ‘C’
SCERT Maharashtra solutions for Chemistry [English] 12 Standard HSC 10 Halogen Derivatives Long Answer Questions
4 Marks
Write the equations for the preparation of ethyl chloride using
- Hydrogen halide
- ethene
- Thionyl chloride
Which of these methods is preferred and why?
What is dehydrohalogenation? State the rule for the formation of the preferred product of dehydrohalogenation.
Predict all the alkenes that would be formed by dehydrohalogenation of the following alkyl halide.
- 2-chloro-2-methylbutane
- 3-bromo-2,2,3-trimethylpentane
Observe the following compounds and answer the questions given below.
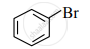
(I)
\[\ce{\underset{\text{(II)}}{CH3 - CH2 - Br}}\]
- Identify the type of halides.
- Explain the nature of the C – Br bond in both of these halides.
- Which of these compounds will undergo aqueous alkaline hydrolysis readily? Write the reaction in support of your answer.
Solutions for 10: Halogen Derivatives
![SCERT Maharashtra solutions for Chemistry [English] 12 Standard HSC chapter 10 - Halogen Derivatives SCERT Maharashtra solutions for Chemistry [English] 12 Standard HSC chapter 10 - Halogen Derivatives - Shaalaa.com](/images/chemistry-english-12-standard-hsc_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
SCERT Maharashtra solutions for Chemistry [English] 12 Standard HSC chapter 10 - Halogen Derivatives
Shaalaa.com has the Maharashtra State Board Mathematics Chemistry [English] 12 Standard HSC Maharashtra State Board solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. SCERT Maharashtra solutions for Mathematics Chemistry [English] 12 Standard HSC Maharashtra State Board 10 (Halogen Derivatives) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. SCERT Maharashtra textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry [English] 12 Standard HSC chapter 10 Halogen Derivatives are Classification of Halogen Derivatives, Nomenclature of Halogen Derivatives, Methods of Preparation of Alkyl Halides, Physical Properties, Optical Isomerism in Halogen Derivatives, Chemical Properties, Reaction with Active Metals, Uses and Environmental Effects of Some Polyhalogen Compounds, Nomenclature, Reactions of Haloalkanes - Elimination Reactions.
Using SCERT Maharashtra Chemistry [English] 12 Standard HSC solutions Halogen Derivatives exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in SCERT Maharashtra Solutions are essential questions that can be asked in the final exam. Maximum Maharashtra State Board Chemistry [English] 12 Standard HSC students prefer SCERT Maharashtra Textbook Solutions to score more in exams.
Get the free view of Chapter 10, Halogen Derivatives Chemistry [English] 12 Standard HSC additional questions for Mathematics Chemistry [English] 12 Standard HSC Maharashtra State Board, and you can use Shaalaa.com to keep it handy for your exam preparation.
