Advertisements
Advertisements
प्रश्न
A reaction is first order in A and second order in B. How is the rate affected when the concentrations of both A and B are doubled?
उत्तर
When the concentrations of both A and B are doubled,
Rate = k(2a) (2b)2
= 8kab2
Therefore, the rate of reaction will increase 8 times.
APPEARS IN
संबंधित प्रश्न
A → B is a first order reaction with rate 6.6 × 10-5m-s-1. When [A] is 0.6m, rate constant of the reaction is
- 1.1 × 10-5s-1
- 1.1 × 10-4s-1
- 9 × 10-5s-1
- 9 × 10-4s-1
From the rate expression for the following reaction, determine the order of reaction and the dimension of the rate constant.
\[\ce{H2O2_{( aq)} + 3I^-_{( aq)} + 2H^+ -> 2H2O_{(l)} + I^-_3}\] Rate = k[H2O2][I−]
In a reaction between A and B, the initial rate of reaction (r0) was measured for different initial concentrations of A and B as given below:
| A/mol L−1 | 0.20 | 0.20 | 0.40 |
| B/mol L−1 | 0.30 | 0.10 | 0.05 |
| r0/mol L−1 s−1 | 5.07 × 10−5 | 5.07 × 10−5 | 1.43 × 10−4 |
What is the order of the reaction with respect to A and B?
Rate of reaction for the combustion of propane is equal to:
\[\ce{C3H8_{(g)} + 5O2_{(g)} -> 3CO2_{(g)} + 4H2O_{(g)}}\]
Which of the following statement is true for order of a reaction?
Which of the following statements is not correct about order of a reaction.
Consider the reaction A ⇌ B. The concentration of both the reactants and the products varies exponentially with time. Which of the following figures correctly describes the change in concentration of reactants and products with time?
For which type of reactions, order and molecularity have the same value?
In a reaction if the concentration of reactant A is tripled, the rate of reaction becomes twenty seven times. What is the order of the reaction?
Why molecularity is applicable only for elementary reactions and order is applicable for elementary as well as complex reactions?
Why can we not determine the order of a reaction by taking into consideration the balanced chemical equation?
Match the graph given in Column I with the order of reaction given in Column II. More than one item in Column I may link to the same item of Column II.
| Column I | Column II | |
| (i) | 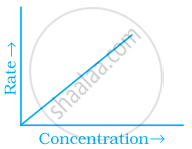 |
|
| (ii) | 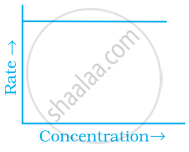 |
(a) 1st order |
| (iii) | 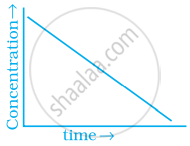 |
(b) Zero-order |
| (iv) | 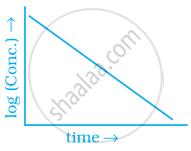 |
A catalyst in a reaction changes which of the following?
The role of a catalyst is to change
The rate constant for the reaction \[\ce{2H2O5 -> 4NO2 + O2}\] is 30 × 10–5 sec–1. if the rate is 204 × 10–5 mol L–1 S–1, then the concentration of N2O5 (in mol–1) is-
The following data was obtained for chemical reaction given below at 975 K.
\[\ce{2NO(g) + 2H2(g) -> N2(g) + 2H2O(g)}\]
| [NO] | [H2] | Rate | |
| Mol L-1 | Mol L-1 | Mol L-1 s-1 | |
| (1) | 8 × 10-5 | 8 × 10-5 | 7 × 10-9 |
| (2) | 24 × 10-5 | 8 × 10-5 | 2.1 × 10-8 |
| (3) | 24 × 10-5 | 32 × 10-5 | 8.4 × 10-8 |
The order of the reaction with respect to NO is ______. (Integer answer)
For a chemical reaction starting with some initial concentration of reactant At as a function of time (t) is given by the equation,
`1/("A"_"t"^4) = 2 + 1.5 xx 10^-3` t
The rate of disappearance of [A] is ____ × 10-2 M/sec when [A] = 2 M.
[Given: [At] in M and t in sec.]
[Express your answer in terms of 10-2 M /s]
[Round off your answer if required]
Which of the following statement is true?
