Advertisements
Advertisements
प्रश्न
Define the following terms:
Pseudo first-order reaction
उत्तर
Pseudo first-order reaction
Pseudo first-order reaction: The reaction which is bimolecular but whose order is one is called pseudo first-order reaction. This happens when one of the reactants is present in a large amount. For example, in acidic hydrolysis of ester (ethyl acetate), water is present in a large quantity.
CH3COOC2H5 + H2O →→ CH3COOH + C2H5OH
Similarly, inversion of cane sugar or hydrolysis of cane sugar is an example of pseudo first-order reaction.
APPEARS IN
संबंधित प्रश्न
For the hydrolysis of methyl acetate in aqueous solution, the following results were obtained :
| t/s | 0 | 30 | 60 |
| [CH3COOCH3] / mol L–1 | 0.60 | 0.30 | 0.15 |
(i) Show that it follows pseudo first order reaction, as the concentration of water remains constant.
(ii) Calculate the average rate of reaction between the time interval 30 to 60 seconds.
(Given log 2 = 0.3010, log 4 = 0.6021)
For a chemical reaction R → P, the variation in the concentration (R) vs. time (t) plot is given as:
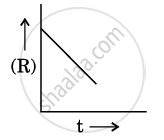
(i) Predict the order of the reaction.
(ii) What is the slope of the curve ?
(iii) Write the unit of rate constant for this reaction.
A reaction is first order in A and second order in B. How is the rate affected on increasing the concentration of B three times?
Write resonating structures of ozone.
Which of the following statements is not correct about order of a reaction.
Why can’t molecularity of any reaction be equal to zero?
Assertion: Order and molecularity are same.
Reason: Order is determined experimentally and molecularity is the sum of the stoichiometric coefficient of rate determining elementary step.
For a reaction A + B → products, the rate law is given by: r = `K[A]^(1/2)`. What is the order of reaction?
At concentration of 0.1 and 0.2 mol L–1 the rates of deem position of a compound were found to be 0.18 and 0.72 mol L–1 m–1. What is the order of the reaction?
Identify the order of reaction from the following unit for its rate constant:
L mol–1s–1
