Advertisements
Advertisements
Question
Define the following terms:
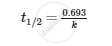
Half-life period of reaction (t1/2).
Solution
For zero-order reaction,
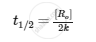
APPEARS IN
RELATED QUESTIONS
Write molecularity of the following reaction:
2NO(g)+O2(g)→2NO2(g)
For a reaction : 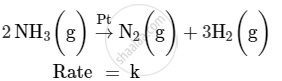
(i) Write the order and molecularity of this reaction.
(ii) Write the unit of k.
A reaction is first order in A and second order in B. Write the differential rate equation.
Molecularity of a reaction _____________.
Which of the following statements is not correct about order of a reaction.
Identify the order of reaction from the following unit for its rate constant:
L mol–1s–1
The conversion of molecules A to B follow second order kinetics. If concentration of A is increased to three times, how will it affect the rate of formation of B?
On heating compound (A) gives a gas (B) which is constituent of air. The gas when treated with H2 in the presence of catalyst gives another gas (C) which is basic in nature, (A) should not be ______.
A drop of solution (volume 0.05 ml) contains 3.0 × 10-6 mole of H+. If the rate constant of disappearance of H+ is 1.0 × 107 mole l-1s-1. It would take for H+ in drop to disappear in ______ × 10-9s.
A reaction is second order with respect to a reactant. How is the rate of reaction affected if the concentration of the reactant is reduced to half?
