Advertisements
Advertisements
Question
Identify the type of overlap present in H-F molecule. Explain diagrammatically.
Solution
s–p σ overlap:
- In this type of overlap one half-filled s orbital of one atom and one half-filled p orbital of another orbital overlap along the internuclear axis.
e.g. Formation of HF molecule by s–p overlap:
Hydrogen atom (Z = 1) has electronic configuration: 1s1 and fluorine atom (Z = 9) has electronic configuration 1s2 2s2 \[\ce{2p^2_{{x}}}\] \[\ce{2p^2_{{y}}}\] \[\ce{2p^1_{{z}}}\]. During the formation of HF molecule, half-filled 1s orbital of hydrogen atom overlaps coaxially with half-filled 2pz orbital of fluorine atom with opposite electron spin and an s–p σ bond is formed. - Diagram:

APPEARS IN
RELATED QUESTIONS
Draw an orbital diagram of Fluorine molecule
Distinguish between sigma and pi bond.
Display electron distribution around the oxygen atom in the water molecule and state the shape of the molecule, also write the H-O-H bond angle.
Give reasons for need of Hybridization
Explain geometry of methane molecule on the basis of Hybridization.
Give a reason for carbon is tetravalent in nature.
Identify the type of overlap present in H2. Explain diagrammatically.
Complete the following Table.
| Molecule | Type of Hybridization | Type of bonds | Geometry | Bond angle |
| CH4 | - | 4C-H 4σ bonds |
Tetrahedral | - |
| NH3 | sp3 | 3N-H 3σ bonds 1 lone pair |
- | - |
| H2O | - | - | angular | 104.5° |
| BF3 | sp2 | - | - | 120° |
| C2H4 | - | - | - | 120° |
| BeF2 | - | 2 Be-F | Linear | - |
| C2H2 | sp | (3σ+2π) 1C-C σ 2C-H σ 2C-C π |
- | - |
Give the type of overlap by which the pi (π) bond is formed.
The ratio of number of sigma (σ) and pi (л) bonds in 2- butynal is ______.
The correct order of O – O bond length in hydrogen peroxide, ozone and oxygen is
XeF2 is isostructural with ______.
What is a pi - bond?
Which bond is stronger σ or π? Why?
Considering x-axis as the molecular axis which out of the following will form a sigma bond.
1s and 2py
Considering x-axis as the molecular axis which out of the following will form a sigma bond.
2px and 2py
Considering x-axis as the molecular axis which out of the following will form a sigma bond.
2px and 2pz
Which of the following molecule contain 50% p-character of hybrid orbital in C atom?
Which of the following is correct decreasing order of the repulsive Interaction of electron pairs in a molecule?
The overlap of orbitals involved in the formation of C - Br bond in vinyl bromide is ______.
If the electronic configuration of an element is 1s2 2s2 2p6 3s2 3p6 3d2 4s2, the four electrons involved in chemical bond formation will be ______.
Why does type of overlap given in the following figure not result in bond formation?
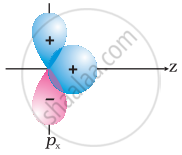 |
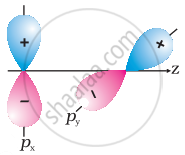 |
Briefly describe the valence bond theory of covalent bond formation by taking an example of hydrogen. How can you interpret energy changes taking place in the formation of dihydrogen?
Match List - I with List - II.
| List - I | List - II | ||
| (a) | \[\ce{PCl5}\] | (i) | Square pyramidal |
| (b) | \[\ce{SF6}\] | (ii) | Trigonal planar |
| (c) | \[\ce{BrF5}\] | (iii) | Octahedral |
| (d) | \[\ce{BF3}\] | (iv) | Trigonal bipyramidal |
Choose the correct answer from the options given below.
The \[\ce{H - N - H}\] bond angle in ammonia molecule is ______.
