Advertisements
Advertisements
Question
When 3-methylbutan-2-ol is treated with HBr, the following reaction takes place:
\[\begin{array}{cc}
\phantom{.......................}\ce{Br}\\
\phantom{......................}|\\
\ce{CH3 - CH - CH - CH3 ->[HBr] CH3 - C - CH2 - CH3}\\
\phantom{.}|\phantom{......}|\phantom{......................}|\phantom{........}\\
\phantom{}\ce{CH3}\phantom{...}\ce{OH}\phantom{...................}\ce{CH3}\phantom{.....}
\end{array}\]
Give a mechanism for this reaction.
(Hint: The secondary carbocation formed in step II rearranges to a more
stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.)
Solution
The described reaction is an example of carbocation rearrangement that occurs via hydride shift. The mechanism for it is –
Step 1: Formation of carbocation: Protonation of alcohol.
\[\ce{HBr -> H+ + B\overset{—}{r}}\]
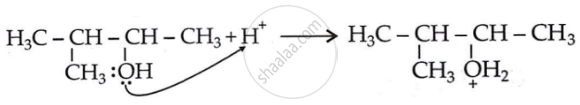
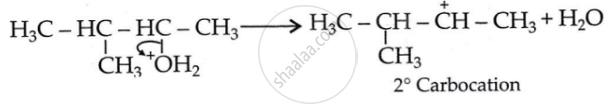
Step 2: 1, 2-hydride shift: Formation of a more stable, 3° carbocation.
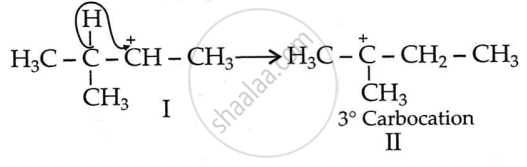
Initially, a 2° carbocation (I) was produced. However, the more stable 3° counterpart causes a hydride shift, forming the more stable carbocation (II).
Step 3: Attack of nucleophile: Generation of product.
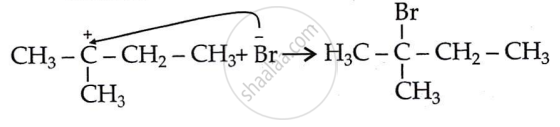
APPEARS IN
RELATED QUESTIONS
Show how will you synthesize pentan-1-ol using a suitable alkyl halide.
Give the equation of the following reaction:
Oxidation of propan-1-ol with alkaline KMnO4 solution.
Give the equation of the following reaction:
Treating phenol with chloroform in the presence of aqueous NaOH.
Name the reagent used in the following reaction:
Oxidation of a primary alcohol to aldehyde.
The compound which reacts fastest with Lucas reagent at room temperature is:
Which of the following are used to convert RCHO into RCH2OH?
(i) H2/Pd
(ii) LiAlH4
(iii) NaBH4
(iv) Reaction with RMgX followed by hydrolysis
Which one of the following on oxidation gives a ketone?
Identify the secondary alcohols from the following set:
- \[\ce{CH3CH2CH(OH)CH3}\]
- \[\ce{(C2H5)3COH}\]
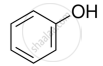
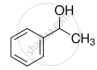
The process of converting alkyl halides into alcohols involves ______.
Mark the correct increasing order of reactivity of the following compounds with HBr/HCl.
| (a) | 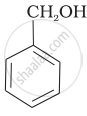 |
| (b) | 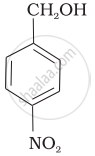 |
| (c) | 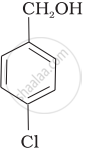 |
Suggest a reagent for the following conversion.

Alcohols react with active metals e.g. Na, K etc. to give corresponding alkoxides. Write down the decreasing order of reactivity of sodium metal towards primary, secondary and tertiary alcohols.
In Kolbe’s reaction, instead of phenol, phenoxide ion is treated with carbon dioxide. Why?
Assertion: Bond angle in ethers is slightly less than the tetrahedral angle.
Reason: There is a repulsion between the two bulky (–R) groups.
Write the mechanism of acid-catalysed dehydration of ethanol to yield ethene.
Write the mechanism of acid dehydration of ethanol to yield ethene.
Write the mechanism of acid dehydration of ethanol to yield ethene.
Write the mechanism of acid dehydration of ethanol to yield ethene.
Write the mechanism of acid dehydration of ethanol to yield ethene.
