Advertisements
Advertisements
प्रश्न
When we heat an object, it expands. Is work done by the object in this process? Is heat given to the object equal to the increase in its internal energy?
उत्तर
When we heat an object, it expands, i.e. its volume increases.
Work done by the system, ΔW = P Δ V
Using the first law of thermodynamics, we get
ΔQ = ΔU + ΔW
Since the volume changes, ΔW has some non-zero positive value. Thus, heat given to the object is not equal to the increase in the internal energy of the system.
APPEARS IN
संबंधित प्रश्न
Write the mathematical expression of the First Law of Thermodynamics for the Isobaric process.
The first law of thermodynamics is a statement of ____________ .
The pressure of a gas changes linearly with volume from 10 kPa, 200 cc to 50 kPa, 50 cc. (a) Calculate the work done by the gas. (b) If no heat is supplied or extracted from the gas, what is the change in the internal energy of the gas?
Calculate the increase in the internal energy of 10 g of water when it is heated from 0°C to 100°C and converted into steam at 100 kPa. The density of steam = 0.6 kg m−3. Specific heat capacity of water = 4200 J kg−1 °C−1 and the latent heat of vaporization of water = 2.25 × 10 6J kg−1.
A mixture of hydrogen and oxygen is enclosed in a rigid insulating cylinder. It is ignited by a spark. The temperature and pressure both increase considerably. Assume that the energy supplied by the spark is negligible, what conclusions may be drawn by application of the first law of thermodynamics?
A resistor held in running water carries electric current. Treat the resistor as the system
- Does heat flow into the resistor?
- Is there a flow of heat into the water?
- Is any work done?
- Assuming the state of resistance to remain unchanged, apply the first law of thermodynamics to this process.
ΔU is equal to ____________ work.
The isothermal bulk modulus of a perfect gas at pressure P is numerically equal to ____________.
If an average person jogs, hse produces 14.5 × 103 cal/min. This is removed by the evaporation of sweat. The amount of sweat evaporated per minute (assuming 1 kg requires 580 × 103 cal for evaparation) is ______.
Three copper blocks of masses M1, M2 and M3 kg respectively are brought into thermal contact till they reach equilibrium. Before contact, they were at T1, T2, T3 (T1 > T2 > T3). Assuming there is no heat loss to the surroundings, the equilibrium temprature T is (s is specific heat of copper)
Can a system be heated and its temperature remains constant?
Is it possible to increase the temperature of a gas without adding heat to it? Explain.
A cycle followed by an engine (made of one mole of an ideal gas in a cylinder with a piston) is shown in figure. Find heat exchanged by the engine, with the surroundings for each section of the cycle. (Cv = (3/2)R)
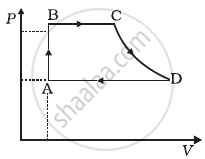
- AB : constant volume
- BC : constant pressure
- CD : adiabatic
- DA : constant pressure
Mathematical equation of first law of thermodynamics for isochoric process is ______.
What work will be done, when 3 moles of an ideal gas are compressed to half the initial volume at a constant temperature of 300 K?
Derive an expression for the work done during an isothermal process.
One mole of an ideal gas is initially kept in a cylinder with a movable frictionless and massless piston at pressure of 1.01MPa, and temperature 27°C. It is then expanded till its volume is doubled. How much work is done if the expansion is isobaric?
Obtain an expression for the workdone by a gas in an isothermal process.
Calculate work done when 2 moles of ideal gas expands by 5 dm3 isothermally at pressure 1.2 bar.
What is an isothermal process?
