Advertisements
Online Mock Tests
Chapters
1: Force
2: Work, Energy and Power
3: Machines
LIGHT
4: Refraction of Light at Plane Surfaces
5: Refraction through a Lens
6: Spectrum
SOUND
7: Sound
ELECTRICITY AND MAGNETISM
8: Current Electricity
9: Household Circuits
10: Electro-Magnetism
HEAT
11: Calorimetry
MODERN PHYSICS
▶ 12: Radioactivity
![Selina solutions for Physics [English] Class 10 ICSE chapter 12 - Radioactivity Selina solutions for Physics [English] Class 10 ICSE chapter 12 - Radioactivity - Shaalaa.com](/images/physics-english-class-10-icse_6:4c973dd038c545c9a2b6db170ad2f542.jpg)
Advertisements
Solutions for Chapter 12: Radioactivity
Below listed, you can find solutions for Chapter 12 of CISCE Selina for Physics [English] Class 10 ICSE.
Selina solutions for Physics [English] Class 10 ICSE 12 Radioactivity EXERCISE - 12(A) [Pages 297 - 300]
Name the three constituents of an atom and state their mass and charge of each. How are they distributed in an atom?
Define the term atomic number.
Define the term mass number.
What is nucleus of an atom? Compare its size with that of the atom. Name its constituents. How is the number of these constituents determined by the atomic number and mass number of the atom?
State the atomic number and mass number of \[\ce{^23_11Na}\] and draw its atomic model.
Give one example of isotopes.
Give one example of isobars.
What is the name given to elements with the same mass number and different atomic number?
Name the atoms of a substance having same atomic number, but different mass numbers. Give one example of such a substance. How do the structures of such atoms differ?
What is meant by radioactivity?
Name two radioactive substances.
A radioactive substance is oxidized. What changes would you expect to take place in the nature of radioactivity? Explain your answer.
A radioactive source emits three types of radiations.
Name the three radiations.
A radioactive source emits three types of radiations.
Name the radiations which are deflected by the electric field.
A radioactive source emits three types of radiations.
Name the radiation which is most penetrating.
A radioactive source emits three types of radiations.
Name the radiation which travels with the speed of light.
A radioactive source emits three types of radiations.
Name the radiation which has the highest ionizing power.
A radioactive source emits three types of radiations.
Name the radiation consisting of the same kind of particles as the cathode rays.
A radioactive source emits three types of radiations.
Which radiation has zero mass?
A radioactive source emits three types of radiations.
Name the radiation which has the lowest ionizing power.
A radioactive source emits three types of radiations.
Name the radiation which has the lowest penetrating power.
A radioactive source emits three type of radiations. Give the charge and mass of particles composing the alpha radiations.
A radioactive source emits three type of radiations.
When the alpha particle becomes neutral, it is found to be the atom of a rare gas. Name this rare gas and draw a model of its neutral atom.
A radioactive source emits three type of radiations.
From which part of the atom do these radiations come?
The diagram in figure shows a radioactive source S placed in a thick lead walled container. The radiations given out are allowed to pass through a magnetic field. The magnetic field (shown as ×) acts perpendicular to the plane of paper inwards. Arrows shows the paths of the radiation A, B and C.
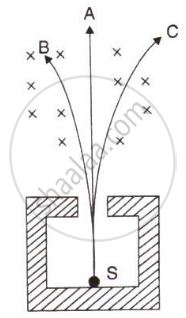
- Name the radiations labelled A, B and C.
- Explain clearly how you used the diagram to arrive at the answer in part (a).
In following Figure shows a mixed source R of alpha and beta particles in a thick lead walled container. The particles pass through a magnetic field in a direction perpendicular to the plane of paper inwards as shown by ×.
- Show in the diagram how the particles get affected.
- Name the law used in part (a).
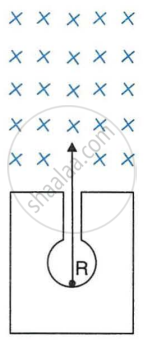
[Hint: Alpha particles will deflect to the left while beta particles to the right]
In following Figure shows a radioactive source S in a thick lead walled container having a narrow opening. The radiations pass through an electric field between the plates A and B.
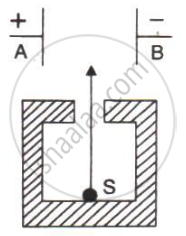
- Complete the diagram to show the paths of α, β and γ radiations.
- Why is the source S kept in a thick lead walled container with a narrow opening?
- Name the radiation which is unaffected by the electrostatic field.
- Which radiation is defleced the most. Given reason.
- Which among the three radiations causes the least biological damage?
Explain why alpha and beta particles are deflected in an electric or a magnetic field, but gamma rays are not deflected in such a field.
Is it possible to deflect γ - radiations in a way similar to α and β -particles, using the electric or magnetic field? Give reasons.
State following four properties each of α, β and γ radiations:
- Nature,
- Charge,
- Mass and
- Effect of electric field.
Arrange the α, β, and γ raditions in ascending order of their ionising powers.
Arrange the α, β and γ radiations in ascending order of their penetrating power.
State the speed of each of α, β and γ radiations.
What is the composition of α, β and γ radiations?
Is it possible for a hydrogen \[\ce{^1_1H}\] nucleus to emit an alpha particle ? Give a reason for your answer.
Which one α, β and γ has the least penetrating power?
How γ - radiations are produced?
Mention two common properties of the gamma radiations and visible light.
An α - particle captures one electron. In this case, what does it change to?
An α - particle captures two electrons. In each case, what does it change to?
What kind of change takes place in a nucleus when a β - particle is emitted? Express it by an equation. State whether
- atomic number and
- mass number are conserved in a radioactive β - decay?
A certain radioactive nucleus emits a particle that leaves its mass unchanged, but increased its atomic number by one. Identify the particle and write its symbol.
What happens to the atomic number of element when (i) An α -particle, (ii) A β -particle 1 and (iii) γ-radiation is emitted?
What happens to the mass number of an element when (i) an α -particle, (ii) a β -particle, and (iii) γ -radiation is emitted?
What happens to the position of an element in the periodic table when its nucleus emits an α - particle. Give reason for your answer.
What happens to the position of an element in the periodic table when its nucleus emits β -particle? Give reasons for your answer.
What happens to the position of an element in the periodic table when its nucleus emits γ radiation? Give reason for your answer.
What changes occur in the nucleus of a radioactive element when it emits an alpha particle. Give one example, in support of your answer.
What changes occur in the nucleus of a radioactive element when it emits a beta particle. Give one example, in support of your answer.
What changes occur in the nucleus of a radioactive element when it emits gamma radiation? Give one example, in support of your answer.
An atomic nucleus A is composed of 84 protons and 128 neutrons.
- The nucleus A emits an alpha particle and is transformed into nucleus B. What is the composition of nucleus B?
- The nucleus B emits a beta particle is transformed into nucleus C. What is the composition of nucleus C?
- Does the composition of nucleus C change if it emits gamma radiations?
A certain nucleus A (mass number 238 and atomic number 92) is radioactive and becomes a nucleus B (mass number 234 and atomic number 90) by the emission of a particle.
- Name the particle emitted.
- Explain how you arrived at your answer.
- State the change in the form of a reaction.
State whether the following nuclear disintegration are allowed or not (star indicate an exited state). Give reason if it is not allowed.
\[\ce {^A_Z X^* -> ^A_ZX + γ}\]
State whether the following nuclear disintegration are allowed or not (star indicate an exited state). Give reason if it is not allowed.
\[\ce{^A_Z X -> ^A_{Z-2} X + ^4_2 He}\]
A nucleus is \[\ce {^24_11 Na}\] is β-radioactive.
What are the numbers 24 and 11 called?
A nucleus \[\ce{^24_11Na}\] is β-radioactive.
Write the equation representing β-decay.
A nucleus is \[\ce {^24_11 Na} \] β-radioactive.
What general name is given to the product nucleus with respect to \[\ce{^24_11 Na}\]?
A nucleus of stable phosphorus has 15 protons and 16 neutrons.
- What is its atomic number and mass number?
- The nucleus of radio phosphorous has one neutron more than the stable nucleus. What will be its atomic number and mass number?
- What will be the atomic number and mass number of new nucleus formed by decay of a β-particle by the radio phosphorus in part (b)?
An element P disintegrates by α-emission and the new element suffers two further disintegration, both by β-emission, to form an element Q. Explain that fact P and Q are isotopes.
A nucleus \[\ce {^A_Z X}\] emits 2 α particles and 1 β particles to form a nucleus \[\ce {^222_85 R}\]. Find the atomic nucleus and mass number of X.
Complete the following sentences:
The mass number and atomic number of an element are not changed when it emits ______.
The atomic number of a radioactive element is not changed when it emits ______.
During the emission of a beta particle, the ______ number remains same.
Complete the following nuclear change:
\[\ce {^a_x P -> Q + ^0_-1β}\]
Complete the following nuclear change:
\[\ce{^238_92U -> ^234_90Th}\] + ______ + energy
Complete the following nuclear change:
\[\ce {^238_92 P \text ->[α] Q \text ->[β] R \text ->[β] S}\]
Complete the following nuclear change:
\[\ce {^A_Z X \text ->[α] X_1 \text ->[γ] X_2 \text ->[2β] X_3}\]
Complete the following nuclear changes:
\[\ce {X \text ->[β] X_1 \text ->[α] X_2 \text ->[α] ^172_69 X_3}\]
What are radio isotopes?
Give one example of a radio isotope.
State one use of radio isotopes.
Why are the alpha particles not used in radio therapy?
Why do we usually use isotopes emitting gamma radiations as radioactive tracers in medical science?
When does the nucleus of an atom tend to become radioactive?
Which of the following is the radio isotope in pair?
\[\ce {^12_6 C , ^14_6 C}\]
Give reason for your answer.
Which of the following is the radio isotope in pair?
\[\ce {^30_15 P , ^32_15 P}\]
Give reason for your answer.
Which of the following is the radio isotope in pair?
\[\ce {^39_19 K , ^40_19 K}\]
Give reason for your answer.
State the medical use of radioactivity.
Arrange the α, β, and γ radiation in ascending order of their biological damage. Give reason.
Name two main sources of nuclear radiations. How are these radiations harmful?
State two safety measures to be taken while establishing a nuclear power plant.
What is meant by nuclear waste?
Suggest one effective way for the safe disposal of nuclear waste.
Name two sources of background radiations.
Is it possible for us to keep ourselves away from background radiations?
MULTIPLE CHOICE TYPE
A radioactive substance emits radiations ______.
α , β and γ simultaneously
in the order α , β and γ one by one
X-ray and Y-ray
α or β
In β-emission from a radioactive substance, an electron is ejected. This electron comes from ______.
the outermost orbit of an atom
the inner orbits of an atom
the surface of substance
the nucleus of an atom
The least penetrating radiation is ______.
α - particles
β - particles
X - rays
γ - radiations
The radiation suffering the maximum deflection in a magnetic field is ______.
α - particles
β - particles
X - rays
γ - radiations
Selina solutions for Physics [English] Class 10 ICSE 12 Radioactivity EXERCISE - 12(B) [Page 306]
What do you mean by nuclear energy? What is responsible for its release?
Write down the Einstein's mass-energy equivalence relation, explaining the meaning of each symbol used in it.
What is a.m.u?
Express 1 a.m.u. in MeV.
Write the approximate mass of a proton, neutron and electron in a.m.u.
What is nuclear fission?
Name the substance used for nuclear fission.
Write one fission reaction.
- Name two isotopes of uranium which are fissionable.
- Which of the isotope mentioned in part (a) above is easily fissionable? Give reason.
- State whether the neutron needed for fission reaction of the isotope mentioned in part (b) above, is slow or fast?
Write the approximate value of the energy released in the fission of one nucleus of \[\ce {^235_92 U}\]. What is the reason for it?
Complete the following nuclear fission reactions:
Complete the following nuclear fission reaction:
\[\ce{^235_92 U + ^1_0n ->_56Ba + ^92Kr + 3 ^1_0 n +}\]______.
Complete the following nuclear fission reaction:
\[\ce {^235_92 U + ^1_0n -> ^148 La + ^85_35 Br +\underline{ } ^1_0n + Energy}\]
What do you mean by the chain reaction in nuclear fission?
How is the chain reaction in nuclear fission controlled?
State two uses of nuclear fission.
Give two differences between the radioactive decay and nuclear fission.
What is nuclear fusion?
Give one example of nuclear fusion.
Write nuclear reaction of nuclear fusion.
What other name is given to nuclear fusion? Give reason.
Why is a very high temperature required for the process of nuclear fusion? State the approximate temperature required.
- Write one nuclear fusion reaction.
- State the approximate value of energy released in the reaction mentioned in part (a).
- Give reason for the release of energy stated in part (b).
Complete the following fusion reaction:
\[\ce {^3_2He + ^2_1H -> ^\underline{}_2He + ^\underline{}_1H + energy}\]
Complete the following fusion reaction:
\[\ce {^2_1H + ^2_1H -> ^\underline{}_2He + ^1_\underline{}n + energy}\]
Name the process, nuclear fission or nuclear fusion, in which the energy released per unit mass is more?
Name the process, fission or fusion which is possible at ordinary temperature.
State one similarity in the process of nuclear fission or fusion.
State two difference between the process of nuclear fission and fusion.
Give two examples of nuclear fusion.
What is the source of energy of sun or stars?
Name the following nuclear reaction:
\[\ce {^235_92U +^1_0n-> ^90_38Sr + ^143_54Xe + 3^1_0n + γ}\]
Name the following nuclear reaction:
\[\ce {^3_1H + ^2_1H -> ^4_2He + ^1_0n + γ}\]
MULTIPLE CHOICE TYPE
The particle used in nuclear fission for bombardment is ______.
alpha particle
proton
beta particle
neutron
The temperature required for the process of nuclear fusion is nearly ______.
1000 K
104K
105K
107K
NUMERICALS
In fission of one uranium - 235 nucleus, the loss in mass is 0.2 a.m.u. Calculate the energy released.
When four hydrogen nuclei combine to form a helium nucleus in the interior of sun, the loss in mass is 0.0265 a.m.u. How much energy is released?
Solutions for 12: Radioactivity
![Selina solutions for Physics [English] Class 10 ICSE chapter 12 - Radioactivity Selina solutions for Physics [English] Class 10 ICSE chapter 12 - Radioactivity - Shaalaa.com](/images/physics-english-class-10-icse_6:4c973dd038c545c9a2b6db170ad2f542.jpg)
Selina solutions for Physics [English] Class 10 ICSE chapter 12 - Radioactivity
Shaalaa.com has the CISCE Mathematics Physics [English] Class 10 ICSE CISCE solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Selina solutions for Mathematics Physics [English] Class 10 ICSE CISCE 12 (Radioactivity) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Selina textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Physics [English] Class 10 ICSE chapter 12 Radioactivity are Isotopes, Isobars, Isotones or Isoneutronic, Radioactivity, Radioactivity as Emission of Alpha, Beta, and Gamma Radiations, Properties of Alpha Particles, Properties of Beta Particles, Properties of Gamma Radiations, Changes Within the Nucleus in Alpha, Beta and Gamma Emission, Alpha Decay (Alpha Emission), Beta Decay (Beta Emission), Gamma Decay (Gamma Emission), Uses of Radioactive Isotopes, Nuclear Energy, Safety Precautions While Using Nuclear Energy, Nuclear Fission, Nuclear Fusion, Distinction Between the Radioactive Decay and Nuclear Fission, Distinction Between the Nuclear Fission and Nuclear Fusion, Structure of the Atom and Nucleus, Atomic Model, Sources of Harmful Radiations, Hazards of Radioactive Substances and Radiation, Background Radiations, Isotopes, Isobars, Isotones or Isoneutronic, Radioactivity, Radioactivity as Emission of Alpha, Beta, and Gamma Radiations, Properties of Alpha Particles, Properties of Beta Particles, Properties of Gamma Radiations, Changes Within the Nucleus in Alpha, Beta and Gamma Emission, Alpha Decay (Alpha Emission), Beta Decay (Beta Emission), Gamma Decay (Gamma Emission), Uses of Radioactive Isotopes, Nuclear Energy, Safety Precautions While Using Nuclear Energy, Nuclear Fission, Nuclear Fusion, Distinction Between the Radioactive Decay and Nuclear Fission, Distinction Between the Nuclear Fission and Nuclear Fusion, Structure of the Atom and Nucleus, Atomic Model, Sources of Harmful Radiations, Hazards of Radioactive Substances and Radiation, Background Radiations.
Using Selina Physics [English] Class 10 ICSE solutions Radioactivity exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Selina Solutions are essential questions that can be asked in the final exam. Maximum CISCE Physics [English] Class 10 ICSE students prefer Selina Textbook Solutions to score more in exams.
Get the free view of Chapter 12, Radioactivity Physics [English] Class 10 ICSE additional questions for Mathematics Physics [English] Class 10 ICSE CISCE, and you can use Shaalaa.com to keep it handy for your exam preparation.
