Topics
Gravitation
- Concept of Gravitation
- Force
- Motion and Rest
- Centripetal Acceleration and Centripetal Force
- Kepler’s Laws
- Newton’s Universal Law of Gravitation
- Uniform Circular Motion (UCM)
- Earth’s Gravitational force
- Acceleration Due to Gravity (Earth’s Gravitational Acceleration)
- Concept of Mass and Weight
- Gravitational Waves
- Free Fall
- Gravitational Potential Energy
- Weightlessness in Space
Periodic Classification of Elements
- History of Periodic Table: Early Attempts at the Classification of Elements
- Dobereiner’s Triads
- Newland's Law of Octaves
- Mendeleev’s Periodic Table
- Merits and Demerits of Mendeleev’s Periodic Table
- Modern Periodic Law
- The Modern Periodic Table
- Structure of the Modern Periodic Table
- Modern Periodic Table and Electronic Configuration of Elements
- Groups and Electronic Configuration
- Periods and Electronic Configuration
- Periodic Properties
- Valency
- Atomic Radius Or Atomic Size
- Metallic and Non-metallic Characters
- Group VIIA Or Group 17 (The Halogens)
Chemical Reactions and Equations
- Chemical Reaction
- Chemical Equation
- Balancing Chemical Equation
- Types of Chemical Change or Chemical Reaction
- Direct Combination (or Synthesis) Reaction
- Decomposition Reactions
- Single Displacement Reactions
- Double Displacement Reaction
- Energy Change in Chemical Reactions
- Rate of Chemical Reaction
- Factors Affecting the Rate of a Chemical Reaction
- Oxidation, Reduction and Redox Reactions
- Corrosion of Metals
- Rancidity of Food and Its Prevention
Effects of Electric Current
- Electric Circuit
- Ohm's Law (V = IR)
- Heating Effect of Electric Current
- Magnetic Effect of Electric Current
- Right-hand Thumb Rule
- Magnetic Field Due to Current in a Loop (Or Circular Coil)
- Magnetic Field Due to a Current Carving Cylindrical Coil (or Solenoid)
- Force on a Current Carrying Conductor in a Magnetic Field
- Fleming’s Left Hand Rule
- Electric Motor
- Electromagnetic Induction
- Galvanometer
- Fleming’s Right Hand Rule
- Types of Current
- Electric Generator
Heat
Refraction of Light
Lenses
- Concept of Lenses
- Spherical Lens
- Convex Lens
- Images Formed by Convex Lenses
- Concave Lens
- Images Formed by Concave Lenses
- Sign Convention
- Lens Formula
- Magnification Due to Spherical Lenses
- Power of a Lens
- Combination of Lenses
- Human Eye
- Working of the Human Eye
- Eye Defect and Its Correction: Myopia Or Near-sightedness
- Eye Defect and its Correction: Hypermetropia or Far-sightedness
- Eye Defect and Its Correction: Presbyopia
- Persistence of Vision
Metallurgy
- Types of Element: Metals
- Physical Properties of Metals
- Chemical Properties of Metal
- Reactions of Metal
- Reactivity Series of Metals
- Types of Element: Non-metal
- Physical Properties of Non-metal
- Chemical Properties of Non-metal
- Ionic Compounds
- Metallurgy
- Basic Principles of Metallurgy
- Extraction of Reactive Metals
- Extraction of Aluminium
- Extraction of Moderately Reactive Metals
- Extraction of Less Reactive Metals
- Refining of Metals
- Corrosion of Metals
- Prevention of Corrosion
Carbon Compounds
- Carbon Compounds in Everyday Life
- Bonds in Carbon Compounds
- Carbon: a Versatile Element
- Properties of Carbon
- Hydrocarbons
- Structural Variations of Carbon Chains in Hydrocarbons
- Functional Groups in Carbon Compounds
- Homologous Series of Carbon Compound
- Nomenclature of Organic Compounds
- The IUPAC System of Nomenclature
- Chemical Properties of Carbon Compounds
- Ethanol
- Ethanoic Acid
- Macromolecules and Polymers
Space Missions
- Concept of Space Missions
- Artificial Satellites
- Types of Satellite
- Orbits of Artificial Satellites
- Space launch technology
- Space Missions Away from Earth
- India’s Space Programmes: Chandrayaan – 1
- India’s Space Programmes: Chandrayaan – 2
- India’s Space Programmes: Chandrayaan – 3
- India’s Space Programmes: Mangalyaan (Mars vehicle)
- India’s Space Programmes: Missions to Other Planets
- India and Space Technology
- Space Debris and Its Management
School of Elements
The Magic of Chemical Reactions
The Acid Base Chemistry
- Properties of Acids
- Strength of Acidic or Basic Solutions
- Strength of Acidic or Basic Solutions
- Acids, Bases and Their Reactivity
- Acid or a Base in a Water Solution
- Preparation and Uses of Baking Soda
- Preparation and Uses of Bleaching Powder
- Preparation and Uses of Washing Soda
- Preparation and Uses of Plaster of Paris
- Chemicals from Common Salt - Soap as a Salt
The Electric Spark
All about Electromagnetism
- Magnetic Force
- The Bar Magnet
- Right-hand Thumb Rule
- Magnetic Field Due to Current in a Loop (Or Circular Coil)
- Magnetic Field Due to a Current Carving Cylindrical Coil (or Solenoid)
- Force on a Current Carrying Conductor in a Magnetic Field
- Electric Motor
- Electromagnetic Induction
- Alternating Current (A.C.) Generator
- Direct Current Motor
- Household Electrical Circuits
Wonders of Light 1
- Spherical Mirrors
- Concave Mirror
- Concave Mirror
- Sign Convention
- Linear Magnification (M) Due to Spherical Mirrors
- Images Formed by Sperical Lenses
- Convex Lens
- Sign Convention
- Magnification Due to Spherical Lenses
- Power of a Lens
- Human Eye
- Eye Defect and Its Correction: Myopia Or Near-sightedness
- Spherical Mirrors
Wonders of Light 2
Striving for better Environment 1
- Pollution and Its Types
- Air Pollution and Its Causes
- Effects of Air Pollution
- Water Pollution and Its Causes
- Effects of Water Pollution
- Soil Pollution and its Causes
- Effects of Soil Pollution
- Noise Pollution
- Radioactive Pollution and Effects
- Abatement of Pollution
- Sustainable Use of Resources
- Bonding in Carbon Compounds
- Covalent Bonding and Electron-Dot Structures
Bonding in Carbon Compounds
Ionic compounds have high melting and boiling points and conduct electricity in molten and dissolved states due to ionic bonds. In contrast, carbon compounds generally have lower melting and boiling points, as shown in the table below, indicating weaker intermolecular forces.
| Compound | Melting Point (°C) | Boiling Point (°C) |
|---|---|---|
| Methane (CH₄) | -183 | -162 |
| Ethanol (CH₃CH₂OH) | -117 | 78 |
| Chloroform (CHCl₃) | -64 | 61 |
| Acetic acid (CH₃COOH) | 17 | 118 |
Most carbon compounds do not conduct electricity, which suggests they lack ionic bonds and do not produce ions in solution.
Electronic Configuration of Carbon
- Atomic number: 6
- Electronic configuration: 2,4
- Valence electrons: 4
- Nearest noble gases: Helium (2), Neon (2,8)
Since carbon has four valence electrons, it can bond in three possible ways to achieve stability:
1. Losing four electrons to form C⁴⁺
- This would require excessive energy, making it unstable.
2. Gaining four electrons to form C⁴⁻
- The carbon nucleus would struggle to hold ten electrons, making this unstable.
3. Sharing four electrons with other atoms (Preferred method)
- Carbon forms covalent bonds, where atoms share electrons instead of transferring them.
Covalent Bonding and Electron-Dot Structures
A covalent bond forms when atoms share electrons to complete their valence shells, making them stable without forming ions. The electron-dot structure represents these shared electrons.
Examples of Covalent Bonding:
1. Hydrogen (H₂)
- Each hydrogen atom has one electron and needs one more to be stable.
- Two hydrogen atoms share electrons, forming a single bond (H—H).

Electron dot structure and line structure of hydrogen molecule with a single bond
2. Oxygen (O₂)
- Each oxygen atom has six valence electrons and needs two more.
- Two oxygen atoms share two pairs of electrons, forming a double bond (O=O).
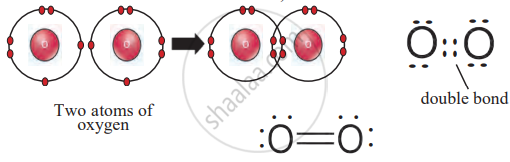
Double Bond
3. Nitrogen (N₂)
- Each nitrogen atom has five valence electrons and needs three more.
- Two nitrogen atoms share three pairs of electrons, forming a triple bond (N≡N).
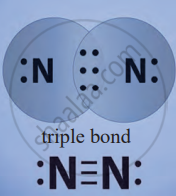
Triple Bond
4. Methane (CH₄)
- Carbon needs four electrons to be stable.
- It shares one electron each with four hydrogen atoms, forming four single covalent bonds.
- Thus, covalent bonding allows carbon to form a wide variety of stable compounds.
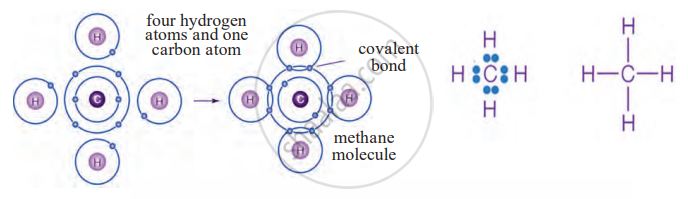
Electron-dot structure and line structure of methane molecule
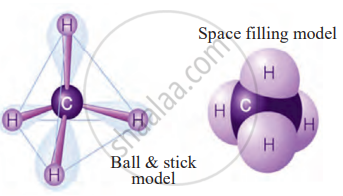
Models of methane molecule
