Topics
Gravitation
- Concept of Gravitation
- Force
- Motion and Rest
- Centripetal Acceleration and Centripetal Force
- Kepler’s Laws
- Newton’s Universal Law of Gravitation
- Uniform Circular Motion (UCM)
- Earth’s Gravitational force
- Acceleration Due to Gravity (Earth’s Gravitational Acceleration)
- Concept of Mass and Weight
- Gravitational Waves
- Free Fall
- Gravitational Potential Energy
- Weightlessness in Space
Periodic Classification of Elements
- History of Periodic Table: Early Attempts at the Classification of Elements
- Dobereiner’s Triads
- Newland's Law of Octaves
- Mendeleev’s Periodic Table
- Merits and Demerits of Mendeleev’s Periodic Table
- Modern Periodic Law
- The Modern Periodic Table
- Structure of the Modern Periodic Table
- Modern Periodic Table and Electronic Configuration of Elements
- Groups and Electronic Configuration
- Periods and Electronic Configuration
- Periodic Properties
- Valency
- Atomic Radius Or Atomic Size
- Metallic and Non-metallic Characters
- Group VIIA Or Group 17 (The Halogens)
Chemical Reactions and Equations
- Chemical Reaction
- Chemical Equation
- Balancing Chemical Equation
- Types of Chemical Change or Chemical Reaction
- Direct Combination (or Synthesis) Reaction
- Decomposition Reactions
- Single Displacement Reactions
- Double Displacement Reaction
- Energy Change in Chemical Reactions
- Rate of Chemical Reaction
- Factors Affecting the Rate of a Chemical Reaction
- Oxidation, Reduction and Redox Reactions
- Corrosion of Metals
- Rancidity of Food and Its Prevention
Effects of Electric Current
- Electric Circuit
- Ohm's Law (V = IR)
- Heating Effect of Electric Current
- Magnetic Effect of Electric Current
- Right-hand Thumb Rule
- Magnetic Field Due to Current in a Loop (Or Circular Coil)
- Magnetic Field Due to a Current Carving Cylindrical Coil (or Solenoid)
- Force on a Current Carrying Conductor in a Magnetic Field
- Fleming’s Left Hand Rule
- Electric Motor
- Electromagnetic Induction
- Galvanometer
- Fleming’s Right Hand Rule
- Types of Current
- Electric Generator
Heat
Refraction of Light
Lenses
- Concept of Lenses
- Spherical Lens
- Convex Lens
- Images Formed by Convex Lenses
- Concave Lens
- Images Formed by Concave Lenses
- Sign Convention
- Lens Formula
- Magnification Due to Spherical Lenses
- Power of a Lens
- Combination of Lenses
- Human Eye
- Working of the Human Eye
- Eye Defect and Its Correction: Myopia Or Near-sightedness
- Eye Defect and its Correction: Hypermetropia or Far-sightedness
- Eye Defect and Its Correction: Presbyopia
- Persistence of Vision
Metallurgy
- Types of Element: Metals
- Physical Properties of Metals
- Chemical Properties of Metal
- Reactions of Metal
- Reactivity Series of Metals
- Types of Element: Non-metal
- Physical Properties of Non-metal
- Chemical Properties of Non-metal
- Ionic Compounds
- Metallurgy
- Basic Principles of Metallurgy
- Extraction of Reactive Metals
- Extraction of Aluminium
- Extraction of Moderately Reactive Metals
- Extraction of Less Reactive Metals
- Refining of Metals
- Corrosion of Metals
- Prevention of Corrosion
Carbon Compounds
- Carbon Compounds in Everyday Life
- Bonds in Carbon Compounds
- Carbon: a Versatile Element
- Properties of Carbon
- Hydrocarbons
- Structural Variations of Carbon Chains in Hydrocarbons
- Functional Groups in Carbon Compounds
- Homologous Series of Carbon Compound
- Nomenclature of Organic Compounds
- The IUPAC System of Nomenclature
- Chemical Properties of Carbon Compounds
- Ethanol
- Ethanoic Acid
- Macromolecules and Polymers
Space Missions
- Concept of Space Missions
- Artificial Satellites
- Types of Satellite
- Orbits of Artificial Satellites
- Space launch technology
- Space Missions Away from Earth
- India’s Space Programmes: Chandrayaan – 1
- India’s Space Programmes: Chandrayaan – 2
- India’s Space Programmes: Chandrayaan – 3
- India’s Space Programmes: Mangalyaan (Mars vehicle)
- India’s Space Programmes: Missions to Other Planets
- India and Space Technology
- Space Debris and Its Management
School of Elements
The Magic of Chemical Reactions
The Acid Base Chemistry
- Properties of Acids
- Strength of Acidic or Basic Solutions
- Strength of Acidic or Basic Solutions
- Acids, Bases and Their Reactivity
- Acid or a Base in a Water Solution
- Preparation and Uses of Baking Soda
- Preparation and Uses of Bleaching Powder
- Preparation and Uses of Washing Soda
- Preparation and Uses of Plaster of Paris
- Chemicals from Common Salt - Soap as a Salt
The Electric Spark
All about Electromagnetism
- Magnetic Force
- The Bar Magnet
- Right-hand Thumb Rule
- Magnetic Field Due to Current in a Loop (Or Circular Coil)
- Magnetic Field Due to a Current Carving Cylindrical Coil (or Solenoid)
- Force on a Current Carrying Conductor in a Magnetic Field
- Electric Motor
- Electromagnetic Induction
- Alternating Current (A.C.) Generator
- Direct Current Motor
- Household Electrical Circuits
Wonders of Light 1
- Spherical Mirrors
- Concave Mirror
- Concave Mirror
- Sign Convention
- Linear Magnification (M) Due to Spherical Mirrors
- Images Formed by Sperical Lenses
- Convex Lens
- Sign Convention
- Magnification Due to Spherical Lenses
- Power of a Lens
- Human Eye
- Eye Defect and Its Correction: Myopia Or Near-sightedness
- Spherical Mirrors
Wonders of Light 2
Striving for better Environment 1
- Pollution and Its Types
- Air Pollution and Its Causes
- Effects of Air Pollution
- Water Pollution and Its Causes
- Effects of Water Pollution
- Soil Pollution and its Causes
- Effects of Soil Pollution
- Noise Pollution
- Radioactive Pollution and Effects
- Abatement of Pollution
- Sustainable Use of Resources
- Nature of the Reactants
- Size of the Particles of Reactants
- Experiment
- Concentration of the Reactants
- Temperature of the Reaction
- Catalyst
Nature of the Reactants
The nature of reactants plays a crucial role in determining the speed of a chemical reaction. Different substances react at different rates based on their reactivity and bonding type.
1. Reactivity of Metals
Let’s compare the reaction of two metals, aluminium (Al) and zinc (Zn), with dilute hydrochloric acid (HCl):
- Both Al and Zn react with HCl, producing hydrogen gas (H₂) and forming water-soluble salts.
- However, aluminium reacts faster than zinc because Al is more reactive than Zn.
- Since aluminium has higher reactivity, it reacts more vigorously, increasing the rate of reaction. Thus, more reactive metals react faster with acids, while less reactive metals react slower.
2. Effect of Bonding Type on Reaction Rate
The type of bonding in reactants also affects the rate of reaction:
- Ionic Compounds: React faster because they involve the direct transfer of ions.
- Covalent Compounds: React slower as their bonds need to be broken before the reaction can occur.
Since ionic bonds involve ion transfer, reactions occur quickly. On the other hand, covalent bonds require bond cleavage, which slows down the reaction. Thus, the reactivity of a substance and its bonding type significantly impact how fast a reaction takes place.
Size of the Particles of Reactants
The size of the reactant particles is another important factor that affects the rate of a chemical reaction.
- When a solid reacts with a liquid or gas, the reaction takes place at the surface of the solid.
- If the solid is broken into smaller pieces or powdered, its surface area increases.
- A larger surface area allows more particles to come in contact with the reactant, increasing the rate of reaction.
Example:
- Powdered zinc (Zn) reacts faster with hydrochloric acid (HCl) than a solid zinc strip because the powder has a greater surface area.
- Fine wood dust catches fire more easily than a solid piece of wood due to increased exposure to oxygen. Thus, smaller particle size leads to a faster reaction rate, while larger particles result in a slower reaction due to reduced surface area.
Experiment
1. Aim: To study how the size of reactant particles affects the rate of a chemical reaction.
2. Requirements: two test tubes, a balance, a measuring cylinder, pieces of Shahabad tile, powdered Shahabad tile, and dilute hydrochloric acid (HCl).
3. Procedure
- Take equal weights of Shahabad tile pieces in one test tube and powdered Shahabad tile in another.
- Add 10 ml of dilute HCl to each test tube.
- Observe the formation of effervescence due to the release of carbon dioxide (CO₂).
4. Observation
- The reaction is slower with Shahabad tile pieces.
- The reaction is faster with the powdered Shahabad tile.
5. Conclusion: Smaller reactant particles increase the surface area, leading to a faster reaction rate.
Concentration of the Reactants
The concentration of reactants plays a crucial role in determining the speed of a chemical reaction. A higher concentration leads to a faster reaction, while a lower concentration slows it down.
For example, when calcium carbonate (CaCO₃) reacts with hydrochloric acid (HCl):
- Dilute HCl reacts slowly with CaCO₃, causing it to disappear gradually and releasing carbon dioxide (CO₂) at a slower rate.
- Concentrated HCl reacts much faster, making CaCO₃ dissolve quickly and releasing CO₂ more rapidly.
This shows that the reaction rate is directly proportional to the concentration of reactants.
- According to collision theory, increasing the concentration of reactants results in more frequent molecular collisions, which speeds up the reaction.
- The law of mass action states that the rate of a chemical reaction is directly proportional to the concentration of the reactants.
- As the reaction progresses, the concentration of reactants decreases, leading to a slower reaction over time.
- A higher concentration of reactants increases the reaction rate by enhancing molecular collisions. Time also plays a significant role, as changes in reactant concentration affect how fast the reaction occurs.
Temperature of the Reaction
Temperature plays a crucial role in determining the speed of a chemical reaction. Generally, increasing the temperature increases the reaction rate, while lowering the temperature slows it down.
For example, in the decomposition of limestone (CaCO₃), lime water does not turn milky before heating because the reaction rate is zero at room temperature. However, once heated, the reaction begins, and carbon dioxide (CO₂) is released, turning the lime water milky.
- When temperature increases, the kinetic energy of reactant molecules also increases.
- This causes more frequent and energetic collisions between molecules.
- A higher proportion of molecules gain the activation energy, leading to more successful reactions.
Increasing temperature speeds up a reaction by providing molecules with more energy for effective collisions. Thus, temperature is a key factor affecting the rate of chemical reactions.
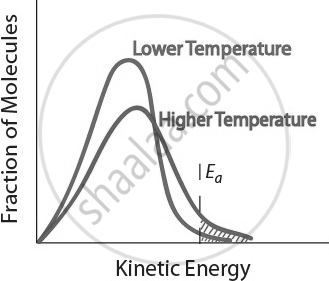
Temperature and Reaction Rate. Effect of temperature on the kinetic energy distribution of molecules in a sample
Catalyst:
A catalyst is a substance that increases the rate of a chemical reaction without undergoing any permanent change itself. It provides an alternative reaction pathway with lower activation energy, allowing reactants to convert into products more easily. Catalysts are essential in many chemical and biological reactions. They speed up reactions by lowering activation energy without being consumed in the process.
Examples,
1. Decomposition of Potassium Chlorate (KClO₃):
- When heated alone, KClO₃ decomposes slowly.
- In the presence of manganese dioxide (MnO₂), the reaction occurs rapidly, releasing oxygen (O₂).
- MnO₂ remains unchanged after the reaction.
2. Decomposition of Hydrogen Peroxide (H₂O₂):
- H₂O₂ decomposes slowly at room temperature.
- Adding MnO₂ speeds up the reaction, breaking H₂O₂ into water and oxygen faster.
Types of Catalysts:
- Positive Catalysts: Increase the reaction rate (e.g., MnO₂ in KClO₃ decomposition).
- Negative Catalysts: Slow down the reaction by increasing activation energy.
