Advertisements
Online Mock Tests
Chapters
2: Kinetic Theory of Gases
3: Calorimetry
4: Laws of Thermodynamics
5: Specific Heat Capacities of Gases
6: Heat Transfer
7: Electric Field and Potential
8: Gauss’s Law
9: Capacitors
10: Electric Current in Conductors
11: Thermal and Chemical Effects of Current
12: Magnetic Field
13: Magnetic Field due to a Current
14: Permanent Magnets
15: Magnetic Properties of Matter
16: Electromagnetic Induction
17: Alternating Current
18: Electromagnetic Waves
19: Electric Current through Gases
20: Photoelectric Effect and Wave-Particle Duality
21: Bohr’s Model and Physics of Atom
22: X-rays
23: Semiconductors and Semiconductor Devices
24: The Nucleus
25: The Special Theory of Relativity
![HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 chapter 1 - Heat and Temperature HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 chapter 1 - Heat and Temperature - Shaalaa.com](/images/9788177092325-concepts-of-physics-vol-2-english-class-11-and-12_6:cd4e4bfcb8474a60871d8e5659ec4eb9.jpg)
Advertisements
Solutions for Chapter 1: Heat and Temperature
Below listed, you can find solutions for Chapter 1 of CBSE, Karnataka Board PUC HC Verma for Concepts of Physics Vol. 2 [English] Class 11 and 12.
HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 1 Heat and Temperature Short Answers [Page 11]
If two bodies are in thermal equilibrium in one frame, will they be in thermal equilibrium in all frames?
Does the temperature of a body depend on the frame from which it is observed?
It is said that mercury is used in defining the temperature scale because it expands uniformly with temperature. If the temperature scale is not yet defined, is it logical to say that a substance expands uniformly with temperature?
In defining the ideal gas temperature scale, it is assumed that the pressure of the gas at constant volume is proportional to the temperature T. How can we verify whether this is true or not? Do we have to apply the kinetic theory of gases? Do we have to depend on experimental result that the pressure is proportional to temperature?
Can the bulb of a thermometer be made of an adiabatic wall?
Why do marine animals live deep inside a lake when the surface of the lake freezes?
The length of a brass rod is found to be less on a hot summer day than on a cold winter day as measured by the same aluminium scale. Can we conclude that brass shrinks on heating?
If mercury and glass had equal coefficients of volume expansion, could we make a mercury thermometer in a glass tube?
The density of water at 4°C is supposed to be 1000 kg m–3. Is it same at sea level and at high altitude?
A tightly closed metal lid of a glass bottle can be opened more easily if it is put in hot water for some time. Explain.
If an automobile engine is overheated, it is cooled by pouring water on it. It is advised that the water should be poured slowly with the engine running. Explain the reason.
Is it possible for two bodies to be in thermal equilibrium if they are not in contact?
A spherical shell is heated. The volume changes according to the equation Vθ = V0 (1 + γθ). Does the volume refer to the volume enclosed by the shell or the volume of the material making up the shell?
HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 1 Heat and Temperature MCQ [Pages 11 - 12]
A system X is neither in thermal equilibrium with Y nor with Z. The systems Y and Z
must be in thermal equilibrium
cannot be in thermal equilibrium
may be in thermal equilibrium
Which of the curves in the following figure represents the relation between Celsius and Fahrenheit temperatures?
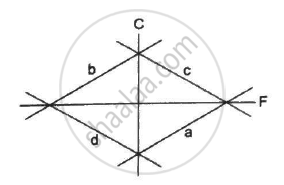
a
b
c
d
Which of the following pairs may give equal numerical values of the temperature of a body?
Fahrenheit and Kelvin
Celsius and Kelvin
Kelvin and Platinum
For a constant-volume gas thermometer, one should fill the gas at
low temperature and low pressure
low temperature and high pressure
high temperature and low pressure
high temperature and high pressure
Consider the following statements.
(A) The coefficient of linear expansion has dimension K–1.
(B) The coefficient of volume expansion has dimension K–1.
A and B are correct.
A is correct but B is wrong.
B is correct but A is wrong.
A and B are wrong.
A metal sheet with a circular hole is heated. The hole
gets larger
gets smaller
retains its size
is deformed
Two identical rectangular strips, one of copper and the other of steel, are riveted together to form a bimetallic strip (acopper> asteel). On heating, this strip will
remain straight
bend with copper on convex side
bend with steel on convex side
get twisted
If the temperature of a uniform rod is slightly increased by ∆t, its moment of inertia I about a perpendicular bisector increases by
zero
αI∆t
2αI∆t
3αI∆t.
If the temperature of a uniform rod is slightly increased by ∆t, its moment of inertia I about a line parallel to itself will increase by
zero
αI∆t
2αI∆t
3αI∆t
The temperature of water at the surface of a deep lake is 2°C. The temperature expected at the bottom is
0 °C
2 °C
4 °C
6 °C
An aluminium sphere is dipped into water at 10°C. If the temperature is increased, the force of buoyancy
will increase
will decrease
will remain constant
may increase or decrease depending on the radius of the sphere
HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 1 Heat and Temperature MCQ [Page 12]
A spinning wheel is brought in contact with an identical wheel spinning at identical speed. The wheels slow down under the action of friction. Which of the following energies of the first wheel decreases?
(a) Kinetic
(b) Total
(c) Mechanical
(d) Internal
A spinning wheel A is brought in contact with another wheel B, initially at rest. Because of the friction at contact, the second wheel also starts spinning. Which of the following energies of the wheel B increases?
(a) Kinetic
(b) Total
(c) Mechanical
(d) Internal
A body A is placed on a railway platform and an identical body B in a moving train. Which of the following energies of B are greater than those of A, as seen from the ground?
(a) Kinetic
(b) Total
(c) Mechanical
(d) Internal
In which of the following pairs of temperature scales, the size of a degree is identical?
(a) Mercury scale and ideal gas scale
(b) Celsius scale and mercury scale
(c) Celsius scale and ideal gas scale
(d) Ideal gas scale and absolute scale
A solid object is placed in water contained in an adiabatic container for some time. The temperature of water falls during this period and there is no appreciable change in the shape of the object. The temperature of the solid object
must have increased
must have decreased
may have increased
may have remained constant
As the temperature is increased, the time period of a pendulum
increases proportionately with temperature
increases
decreases
remains constant
HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 1 Heat and Temperature Exercises [Pages 12 - 14]
The steam point and the ice point of a mercury thermometer are marked as 80° and 20°. What will be the temperature on a centigrade mercury scale when this thermometer reads 32°?
A constant-volume thermometer registers a pressure of 1.500 × 104 Pa at the triple point of water and a pressure of 2.050 × 104 Pa at the normal boiling point. What is the temperature at the normal boiling point?
A gas thermometer measures the temperature from the variation of pressure of a sample of gas. If the pressure measured at the melting point of lead is 2.20 times the pressure measured at the triple point of water, find the melting point of lead.
The pressure measured by a constant volume gas thermometer is 40 kPa at the triple point of water. What will be the pressure measured at the boiling point of water (100°C)?
The pressure of the gas in a constant volume gas thermometer is 70 kPa at the ice point. Find the pressure at the steam point.
The pressures of the gas in a constant volume gas thermometer are 80 cm, 90 cm and 100 cm of mercury at the ice point, the steam point and in a heated wax bath, respectively. Find the temperature of the wax bath.
In a Callender's compensated constant pressure air thermometer, the volume of the bulb is 1800 cc. When the bulb is kept immersed in a vessel, 200 cc of mercury has to be poured out. Calculate the temperature of the vessel.
A platinum resistance thermometer reads 0° when its resistance is 80 Ω and 100° when its resistance is 90 Ω.
Find the temperature at the platinum scale at which the resistance is 86 Ω.
A resistance thermometer reads R = 20.0 Ω, 27.5 Ω, and 50.0 Ω at the ice point (0°C), the steam point (100°C) and the zinc point (420°C), respectively. Assuming that the resistance varies with temperature as Rθ = R0 (1 + αθ + βθ2), find the values of R0, α and β. Here θ represents the temperature on the Celsius scale.
A concrete slab has a length of 10 m on a winter night when the temperature is 0°C. Find the length of the slab on a summer day when the temperature is 35°C. The coefficient of linear expansion of concrete is 1.0 × 10–5 °C–1.
A metre scale made of steel is calibrated at 20°C to give correct reading. Find the distance between the 50 cm mark and the 51 cm mark if the scale is used at 10°C. Coefficient of linear expansion of steel is 1.1 × 10–5 °C–1.
A railway track (made of iron) is laid in winter when the average temperature is 18°C. The track consists of sections of 12.0 m placed one after the other. How much gap should be left between two such sections, so that there is no compression during summer when the maximum temperature rises to 48°C? Coefficient of linear expansion of iron = 11 × 10–6 °C–1.
A circular hole of diameter 2.00 cm is made in an aluminium plate at 0°C. What will be the diameter at 100°C? α for aluminium = 2.3 × 10–5 °C–1.
Two metre scales, one of steel and the other of aluminium, agree at 20°C. Calculate the ratio aluminium-centimetre/steel-centimetre at (a) 0°C, (b) 40°C and (c) 100°C. α for steel = 1.1 × 10–5 °C–1 and for aluminium = 2.3 × 10–5°C–1.
A metre scale is made up of steel and measures correct length at 16°C. What will be the percentage error if this scale is used (a) on a summer day when the temperature is 46°C and (b) on a winter day when the temperature is 6°C? Coefficient of linear expansion of steel = 11 × 10–6 °C–1.
A metre scale made of steel reads accurately at 20°C. In a sensitive experiment, distances accurate up to 0.055 mm in 1 m are required. Find the range of temperature in which the experiment can be performed with this metre scale. Coefficient of linear expansion of steel = 11 × 10–6 °C–1.
The density of water at 0°C is 0.998 g cm–3 and at 4°C is 1.000 g cm–1. Calculate the average coefficient of volume expansion of water in the temperature range of 0 to 4°C.
Find the ratio of the lengths of an iron rod and an aluminium rod for which the difference in the lengths is independent of temperature. Coefficients of linear expansion of iron and aluminium are 12 × 10–6 °C–1 and 23 × 10–6 °C–1 respectively.
A pendulum clock shows correct time at 20°C at a place where g = 9.800 m s–2. The pendulum consists of a light steel rod connected to a heavy ball. It is taken to a different place where g = 9.788 m s–1. At what temperature will the clock show correct time? Coefficient of linear expansion of steel = 12 × 10–6 °C–1.
An aluminium plate fixed in a horizontal position has a hole of diameter 2.000 cm. A steel sphere of diameter 2.005 cm rests on this hole. All the lengths refer to a temperature of 10 °C. The temperature of the entire system is slowly increased. At what temperature will the ball fall down? Coefficient of linear expansion of aluminium is 23 × 10–6 °C–1 and that of steel is 11 × 10–6 °C–1.
A glass window is to be fit in an aluminium frame. The temperature on the working day is 40°C and the glass window measures exactly 20 cm × 30 cm. What should be the size of the aluminium frame so that there is no stress on the glass in winter even if the temperature drops to 0°C? Coefficients of linear expansion for glass and aluminium are 9.0 × 10–6 °C–1 and 24 ×100–6°C–1 , respectively.
The volume of a glass vessel is 1000 cc at 20°C. What volume of mercury should be poured into it at this temperature so that the volume of the remaining space does not change with temperature? Coefficients of cubical expansion of mercury and glass are 1.8 × 10–6 °C–1 and 9.0 × 10–6 °C–1 , respectively.
An aluminium can of cylindrical shape contains 500 cm3 of water. The area of the inner cross section of the can is 125 cm2. All measurements refer to 10°C.
Find the rise in the water level if the temperature increases to 80°C. The coefficient of linear expansion of aluminium is 23 × 10–6 °C–1 and the average coefficient of the volume expansion of water is 3.2 × 10–4 °C–1.
A glass vessel measures exactly 10 cm × 10 cm × 10 cm at 0°C. It is filled completely with mercury at this temperature. When the temperature is raised to 10°C, 1.6 cm3 of mercury overflows. Calculate the coefficient of volume expansion of mercury. Coefficient of linear expansion of glass = 6.5 × 10–1 °C–1.
The densities of wood and benzene at 0°C are 880 kg m3 and 900 kg m–3 , respectively. The coefficients of volume expansion are 1.2 × 10–3 °C–1 for wood and 1.5 × 10–3 °C–1for benzene. At what temperature will a piece of wood just sink in benzene?
A steel rod of length 1 m rests on a smooth horizontal base. If it is heated from 0°C to 100°C, what is the longitudinal strain developed?
A steel rod is clamped at its two ends and rests on a fixed horizontal base. The rod is unstrained at 20°C.
Find the longitudinal strain developed in the rod if the temperature rises to 50°C. Coefficient of linear expansion of steel = 1.2 × 10–5 °C–1.
A steel wire of cross-sectional area 0.5 mm2 is held between two fixed supports. If the wire is just taut at 20°C, determine the tension when the temperature falls to 0°C. Coefficient of linear expansion of steel is 1.2 × 10–5 °C–1 and its Young's modulus is 2.0 × 10–11 Nm–2.
A steel rod is rigidly clamped at its two ends. The rod is under zero tension at 20°C. If the temperature rises to 100°C, what force will the rod exert on one of the clamps? Area of cross-section of the rod is 2.00 mm2. Coefficient of linear expansion of steel is 12.0 × 10–6 °C–1 and Young's modulus of steel is 2.00 × 1011 Nm–2.
Two steel rods and an aluminium rod of equal length l0 and equal cross-section are joined rigidly at their ends, as shown in the figure below. All the rods are in a state of zero tension at 0°C. Find the length of the system when the temperature is raised to θ. Coefficient of linear expansion of aluminium and steel are αa and αs, respectively. Young's modulus of aluminium is Ya and of steel is Ys.
| Steel |
| Aluminium |
| Steel |
A steel ball that is initially at a pressure of 1.0 × 105 Pa is heated from 20°C to 120°C, keeping its volume constant.
Find the pressure inside the ball. Coefficient of linear expansion of steel = 12 × 10–6 °C–1and bulk modulus of steel = 1.6 × 1011 Nm–2.
Show that the moment of inertia of a solid body of any shape changes with temperature as I = I0 (1 + 2αθ), where I0 is the moment of inertia at 0°C and α is the coefficient of linear expansion of the solid.
A torsional pendulum consists of a solid disc connected to a thin wire (α = 2.4 × 10–5°C–1) at its centre. Find the percentage change in the time period between peak winter (5°C) and peak summer (45°C).
A circular disc made of iron is rotated about its axis at a constant velocity ω. Calculate the percentage change in the linear speed of a particle of the rim as the disc is slowly heated from 20°C to 50°C, keeping the angular velocity constant. Coefficient of linear expansion of iron = 1.2 × 10–5 °C–1.
Solutions for 1: Heat and Temperature
![HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 chapter 1 - Heat and Temperature HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 chapter 1 - Heat and Temperature - Shaalaa.com](/images/9788177092325-concepts-of-physics-vol-2-english-class-11-and-12_6:cd4e4bfcb8474a60871d8e5659ec4eb9.jpg)
HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 chapter 1 - Heat and Temperature
Shaalaa.com has the CBSE, Karnataka Board PUC Mathematics Concepts of Physics Vol. 2 [English] Class 11 and 12 CBSE, Karnataka Board PUC solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. HC Verma solutions for Mathematics Concepts of Physics Vol. 2 [English] Class 11 and 12 CBSE, Karnataka Board PUC 1 (Heat and Temperature) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. HC Verma textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Concepts of Physics Vol. 2 [English] Class 11 and 12 chapter 1 Heat and Temperature are Anomalous Expansion of Water, Measurement of Temperature, Ideal-gas Equation and Absolute Temperature, Thermal Expansion, Calorimetry, Change of State - Latent Heat Capacity, Conduction, Convection, Radiation, Newton’s Law of Cooling, Qualitative Ideas of Black Body Radiation, Wien's Displacement Law, Stefan's Law, Liquids and Gases, Thermal Expansion of Solids, Green House Effect, Specific Heat Capacity, Heat and Temperature.
Using HC Verma Concepts of Physics Vol. 2 [English] Class 11 and 12 solutions Heat and Temperature exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in HC Verma Solutions are essential questions that can be asked in the final exam. Maximum CBSE, Karnataka Board PUC Concepts of Physics Vol. 2 [English] Class 11 and 12 students prefer HC Verma Textbook Solutions to score more in exams.
Get the free view of Chapter 1, Heat and Temperature Concepts of Physics Vol. 2 [English] Class 11 and 12 additional questions for Mathematics Concepts of Physics Vol. 2 [English] Class 11 and 12 CBSE, Karnataka Board PUC, and you can use Shaalaa.com to keep it handy for your exam preparation.
