Advertisements
Online Mock Tests
Chapters
2: Kinetic Theory of Gases
3: Calorimetry
4: Laws of Thermodynamics
5: Specific Heat Capacities of Gases
6: Heat Transfer
7: Electric Field and Potential
8: Gauss’s Law
9: Capacitors
10: Electric Current in Conductors
11: Thermal and Chemical Effects of Current
12: Magnetic Field
13: Magnetic Field due to a Current
14: Permanent Magnets
15: Magnetic Properties of Matter
16: Electromagnetic Induction
17: Alternating Current
18: Electromagnetic Waves
19: Electric Current through Gases
▶ 20: Photoelectric Effect and Wave-Particle Duality
21: Bohr’s Model and Physics of Atom
22: X-rays
23: Semiconductors and Semiconductor Devices
24: The Nucleus
25: The Special Theory of Relativity
![HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 chapter 20 - Photoelectric Effect and Wave-Particle Duality HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 chapter 20 - Photoelectric Effect and Wave-Particle Duality - Shaalaa.com](/images/9788177092325-concepts-of-physics-vol-2-english-class-11-and-12_6:cd4e4bfcb8474a60871d8e5659ec4eb9.jpg)
Advertisements
Solutions for Chapter 20: Photoelectric Effect and Wave-Particle Duality
Below listed, you can find solutions for Chapter 20 of CBSE, Karnataka Board PUC HC Verma for Concepts of Physics Vol. 2 [English] Class 11 and 12.
HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 20 Photoelectric Effect and Wave-Particle Duality Short Answers [Page 363]
Can we find the mass of a photon by the definition p = mv?
Is it always true that for two sources of equal intensity, the number of photons emitted in a given time are equal?
What is the speed of a photon with respect to another photon if (a) the two photons are going in the same direction and (b) they are going in opposite directions?
Can a photon be deflected by an electric field? Or by a magnetic field?
A hot body is placed in a closed room maintained at a lower temperature. Is the number of photons in the room increasing?
Should the energy of a photon be called its kinetic energy or its internal energy?
In an experiment on photoelectric effect, a photon is incident on an electron from one direction and the photoelectron is emitted almost in the opposite direction. Does this violate the principle of conservation of momentum?
It is found that yellow light does not eject photoelectrons from a metal. Is it advisable to try with orange light or with green light?
It is found that photosynthesis starts in certain plants when exposed to sunlight, but it does not start if the plants are exposed only to infrared light. Explain.
The threshold wavelength of a metal is λ0. Light of wavelength slightly less than λ0 is incident on an insulated plate made of this metal. It is found that photoelectrons are emitted for some time and after that the emission stops. Explain.
Is p − E/c valid for electrons?
Consider the de-Broglie wavelength of an electron and a proton. Which wavelength is smaller if the two particles have (a) the same speed (b) the same momentum (c) the same energy?
If an electron has a wavelength, does it also have a colour?
HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 20 Photoelectric Effect and Wave-Particle Duality MCQ [Pages 363 - 264]
Planck's constant has the same dimensions as
force × time
force × distance
force × speed
force × distance time
Two photons of
equal wavelength have equal linear momenta
equal energies have equal linear momenta
equal frequencies have equal linear momenta
equal linear momenta have equal wavelengths
Let p and E denote the linear momentum and energy of a photon. If the wavelength is decreased,
both p and E increase
p increases and E decreases
p decreases and E increases
both p and E decrease
Let nr and nb be the number of photons emitted by a red bulb and a blue bulb, respectively, of equal power in a given time.
nr = nb
nr < nb
nr > nb
The information is insufficient to derive a relation between nr and nb.
The equation E = pc is valid
for an electron as well as for a photon
for an electron but not for a photon
for a photon but not for an electron
neither for an electron nor for a photon
The work function of a metal is hv0. Light of frequency v falls on this metal. Photoelectric effect will take place only if
v ≥ v0
v > 2v0
v < v0
v < v0/2
Light of wavelength λ falls on a metal with work-function hc/λ0. Photoelectric effect will take place only if
λ ≥ λ0
λ ≥ 2λ0
λ ≤ λ0
λ < λ0/2
When stopping potential is applied in an experiment on photoelectric effect, no photoelectric is observed. This means that
the emission of photoelectrons is stopped
the photoelectrons are emitted but are re-absorbed
the photoelectrons are accumulated near the collector plate
the photoelectrons are dispersed from the sides of the apparatus
If the frequency of light in a photoelectric experiment is doubled, the stopping potential will ______.
be doubled
be halved
become more than double
become less than double
The frequency and intensity of a light source are doubled. Consider the following statements.
(A) The saturation photocurrent remains almost the same.
(B) The maximum kinetic energy of the photoelectrons is doubled.
A and B are true.
A is true but B is false.
A is false but B is true.
A and B are false.
A point source of light is used in a photoelectric effect. If the source is removed farther from the emitting metal, the stopping potential
will increase
will decrease
will remain constant
will either increase or decrease
A point source causes photoelectric effect from a small metal plate. Which of the following curves may represent the saturation photocurrent as a function of the distance between the source and the metal?

a
b
c
d
A non-monochromatic light is used in an experiment on photoelectric effect. The stopping potential
is related to the mean wavelength
is related to the longest wavelength
is related to the shortest wavelength
is not related to the wavelength
A proton and an electron are accelerated by the same potential difference. Let λe and λpdenote the de Broglie wavelengths of the electron and the proton, respectively.
λe = λp
λe < λp
λe > λp
The relation between λe and λp depends on the accelerating potential difference.
HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 20 Photoelectric Effect and Wave-Particle Duality MCQ [Pages 264 - 364]
When the intensity of a light source in increased,
(a) the number of photons emitted by the source in unit time increases
(b) the total energy of the photons emitted per unit time increases
(c) more energetic photons are emitted
(d) faster photons are emitted
Photoelectric effect supports quantum nature of light because
(a) there is a minimum frequency below which no photoelectrons are emitted
(b) the maximum kinetic energy of photoelectrons depends only on the frequency of light and not on its intensity
(c) even when the metal surface is faintly illuminated the photoelectrons leave the surface immediately
(d) electric charge of the photoelectrons is quantised
A photon of energy hv is absorbed by a free electron of a metal with work-function hv − φ.
The electron is sure to come out.
The electron is sure to come out with kinetic energy hv − φ.
Either the electron does not come out or it comes our with kinetic energy hv − φ.
It may come out with kinetic energy less than hv − φ.
If the wavelength of light in an experiment on photoelectric effect is doubled,
(a) photoelectric emission will not take place
(b) photoelectric emission may or may not take place
(c) the stopping potential will increase
(d) the stopping potential will decrease
The photocurrent in an experiment on photoelectric effect increases if ______.
the intensity of the source is increased
the exposure time is increased
the intensity of the source is decreased
the exposure time is decreased
The collector plate in an experiment on photoelectric effect is kept vertically above the emitter plate. A light source is put on and a saturation photocurrent is recorded. An electric field is switched on that has a vertically downward direction.
The photocurrent will increase.
The kinetic energy of the electrons will increase.
The stopping potential will decrease.
The threshold wavelength will increase.
In which of the following situations, the heavier of the two particles has smaller de Broglie wavelength? The two particles
(a) move with the same speed
(b) move with the same linear momentum
(c) move with the same kinetic energy
(d) have fallen through the same height
HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 20 Photoelectric Effect and Wave-Particle Duality Exercises [Pages 365 - 366]
Visible light has wavelengths in the range of 400 nm to 780 nm. Calculate the range of energy of the photons of visible light.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
Calculate the momentum of a photon of light of wavelength 500 nm.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
An atom absorbs a photon of wavelength 500 nm and emits another photon of wavelength 700 nm. Find the net energy absorbed by the atom in the process.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
Calculate the number of photons emitted per second by a 10 W sodium vapour lamp. Assume that 60% of the consumed energy is converted into light. Wavelength of sodium light = 590 nm
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
When the sun is directly overhead, the surface of the earth receives 1.4 × 103 W m−2 of sunlight. Assume that the light is monochromatic with average wavelength 500 nm and that no light is absorbed in between the sun and the earth's surface. The distance between the sun and the earth is 1.5 × 1011 m. (a) Calculate the number of photons falling per second on each square metre of earth's surface directly below the sun. (b) How many photons are there in each cubic metre near the earth's surface at any instant? (c) How many photons does the sun emit per second?
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A parallel beam of monochromatic light of wavelength 663 nm is incident on a totally reflecting plane mirror. The angle of incidence is 60° and the number of photons striking the mirror per second is 1.0 × 1019. Calculate the force exerted by the light beam on the mirror.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A beam of white light is incident normally on a plane surface absorbing 70% of the light and reflecting the rest. If the incident beam carries 10 W of power, find the force exerted by it on the surface.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A totally reflecting, small plane mirror placed horizontally faces a parallel beam of light, as shown in the figure. The mass of the mirror is 20 g. Assume that there is no absorption in the lens and that 30% of the light emitted by the source goes through the lens. Find the power of the source needed to support the weight of the mirror.
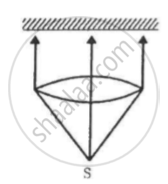
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A 100 W light bulb is placed at the centre of a spherical chamber of radius 20 cm. Assume that 60% of the energy supplied to the bulb is converted into light and that the surface of the chamber is perfectly absorbing. Find the pressure exerted by the light on the surface of the chamber.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A sphere of radius 1.00 cm is placed in the path of a parallel beam of light of large aperture. The intensity of the light is 0.5 W cm−2. If the sphere completely absorbs the radiation falling on it, find the force exerted by the light beam on the sphere.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A sphere of radius 1.00 cm is placed in the path of a parallel beam of light of large aperture. The intensity of the light is 0.5 W cm−2. If the sphere completely absorbs the radiation falling on it, Show that the force on the sphere due to the light falling on it is the same even if the sphere is not perfectly absorbing.
Show that it is not possible for a photon to be completely absorbed by a free electron.
Two neutral particles are kept 1 m apart. Suppose by some mechanism some charge is transferred from one particle to the other and the electric potential energy lost is completely converted into a photon. Calculate the longest and the next smaller wavelength of the photon possible.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
Find the maximum kinetic energy of the photoelectrons ejected when light of wavelength 350 nm is incident on a cesium surface. Work function of cesium = 1.9 eV
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
The work function of a metal is 2.5 × 10−19 J. (a) Find the threshold frequency for photoelectric emission. (b) If the metal is exposed to a light beam of frequency 6.0 × 1014 Hz, what will be the stopping potential?
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
The work function of a photoelectric material is 4.0 eV. (a) What is the threshold wavelength? (b) Find the wavelength of light for which the stopping potential is 2.5 V.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
Find the maximum magnitude of the linear momentum of a photoelectron emitted when a wavelength of 400 nm falls on a metal with work function 2.5 eV.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
When a metal plate is exposed to a monochromatic beam of light of wavelength 400 nm, a negative potential of 1.1 V is needed to stop the photo current. Find the threshold wavelength for the metal.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
In an experiment on photoelectric effect, the stopping potential is measured for monochromatic light beams corresponding to different wavelengths. The data collected are as follows:-
Wavelength (nm): 350 400 450 500 550
Stopping potential (V): 1.45 1.00 0.66 0.38 0.16
Plot the stopping potential against inverse of wavelength (1/λ) on a graph paper and find (a) Planck's constant (b) the work function of the emitter and (c) the threshold wavelength.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
The electric field associated with a monochromatic beam is 1.2 × 1015 times per second. Find the maximum kinetic energy of the photoelectrons when this light falls on a metal surface whose work function is 2.0 eV.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
The electric field associated with a light wave is given by `E = E_0 sin [(1.57 xx 10^7 "m"^-1)(x - ct)]`. Find the stopping potential when this light is used in an experiment on photoelectric effect with the emitter having work function 1.9 eV.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
The electric field at a point associated with a light wave is `E = (100 "Vm"^-1) sin [(3.0 xx 10^15 "s"^-1)t] sin [(6.0 xx 10^15 "s"^-1)t]`.If this light falls on a metal surface with a work function of 2.0 eV, what will be the maximum kinetic energy of the photoelectrons?
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A monochromatic light source of intensity 5 mW emits 8 × 1015 photons per second. This light ejects photoelectrons from a metal surface. The stopping potential for this setup is 2.0 V. Calculate the work function of the metal.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
The figure is the plot of stopping potential versus the frequency of the light used in an experiment on photoelectric effect. Find (a) the ratio h/e and (b) the work function.
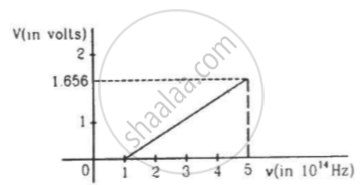
A photographic film is coated with a silver bromide layer. When light falls on this film, silver bromide molecules dissociate and the film records the light there. A minimum of 0.6 eV is needed to dissociate a silver bromide molecule. Find the maximum wavelength of light that can be recorded by the film.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
In an experiment on photoelectric effect, light of wavelength 400 nm is incident on a cesium plate at the rate of 5.0 W. The potential of the collector plate is made sufficiently positive with respect to the emitter, so that the current reaches its saturation value. Assuming that on average, one out of every 106 photons is able to eject a photoelectron, find the photocurrent in the circuit.
A silver ball of radius 4.8 cm is suspended by a thread in a vacuum chamber. Ultraviolet light of wavelength 200 nm is incident on the ball for some time during which light energy of 1.0 × 10−7 J falls on the surface. Assuming that on average, one photon out of every ten thousand is able to eject a photoelectron, find the electric potential at the surface of the ball, assuming zero potential at infinity. What is the potential at the centre of the ball?
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
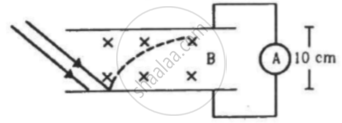
In an experiment on photoelectric effect, the emitter and the collector plates are placed at a separation of 10 cm and are connected through an ammeter without any cell. A magnetic field B exists parallel to the plates. The work function of the emitter is 2.39 eV and the light incident on it has wavelengths between 400 nm and 600 nm. Find the minimum value of B for which the current registered by the ammeter is zero. Neglect any effect of space charge.
In the arrangement shown in the figure, y = 1.0 mm, d = 0.24 mm and D = 1.2 m. The work function of the material of the emitter is 2.2 eV. Find the stopping potential V needed to stop the photocurrent.
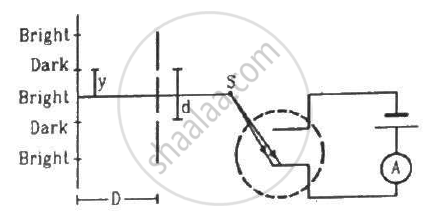
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
In a photoelectric experiment, the collector plate is at 2.0 V with respect to the emitter plate made of copper (φ = 4.5 eV). The emitter is illuminated by a source of monochromatic light of wavelength 200 nm. Find the minimum and maximum kinetic energy of the photoelectrons reaching the collector.
A small piece of cesium metal (φ = 1.9 eV) is kept at a distance of 20 cm from a large metal plate with a charge density of 1.0 × 10−9 C m−2 on the surface facing the cesium piece. A monochromatic light of wavelength 400 nm is incident on the cesium piece. Find the minimum and maximum kinetic energy of the photoelectrons reaching the large metal plate. Neglect any change in electric field due to the small piece of cesium present.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
Consider the situation of the previous problem. Consider the faster electron emitted parallel to the large metal plate. Find the displacement of this electron parallel to its initial velocity before it strikes the large metal plate.
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A horizontal cesium plate (φ = 1.9 eV) is moved vertically downward at a constant speed v in a room full of radiation of wavelength 250 nm and above. What should be the minimum value of v so that the vertically-upward component of velocity is non-positive for each photoelectron?
(Use h = 6.63 × 10-34J-s = 4.14 × 10-15 eV-s, c = 3 × 108 m/s and me = 9.1 × 10-31kg)
A small metal plate (work function φ) is kept at a distance d from a singly-ionised, fixed ion. A monochromatic light beam is incident on the metal plate and photoelectrons are emitted. Find the maximum wavelength of the light beam, so that some of the photoelectrons may go round the ion along a circle.
A light beam of wavelength 400 nm is incident on a metal plate of work function 2.2 eV. (a) A particular electron absorbs a photon and makes two collisions before coming out of the metal. Assuming that 10% of the extra energy is lost to the metal in each collision, find the kinetic energy of this electron as it comes out of the metal. (b) Under the same assumptions, find the maximum number of collisions the electron can suffer before it becomes unable to come out of the metal.
Solutions for 20: Photoelectric Effect and Wave-Particle Duality
![HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 chapter 20 - Photoelectric Effect and Wave-Particle Duality HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 chapter 20 - Photoelectric Effect and Wave-Particle Duality - Shaalaa.com](/images/9788177092325-concepts-of-physics-vol-2-english-class-11-and-12_6:cd4e4bfcb8474a60871d8e5659ec4eb9.jpg)
HC Verma solutions for Concepts of Physics Vol. 2 [English] Class 11 and 12 chapter 20 - Photoelectric Effect and Wave-Particle Duality
Shaalaa.com has the CBSE, Karnataka Board PUC Mathematics Concepts of Physics Vol. 2 [English] Class 11 and 12 CBSE, Karnataka Board PUC solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. HC Verma solutions for Mathematics Concepts of Physics Vol. 2 [English] Class 11 and 12 CBSE, Karnataka Board PUC 20 (Photoelectric Effect and Wave-Particle Duality) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. HC Verma textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Concepts of Physics Vol. 2 [English] Class 11 and 12 chapter 20 Photoelectric Effect and Wave-Particle Duality are Einstein’s Photoelectric Equation: Energy Quantum of Radiation, Particle Nature of Light: The Photon, Photoelectric Effect and Wave Theory of Light, Experimental Study of Photoelectric Effect, Einstein’s Equation - Particle Nature of Light, Electron Emission, Davisson and Germer Experiment, de-Broglie Relation, Wave Nature of Matter, Photoelectric Effect - Hallwachs’ and Lenard’s Observations, Photoelectric Effect - Hertz’s Observations, Dual Nature of Radiation.
Using HC Verma Concepts of Physics Vol. 2 [English] Class 11 and 12 solutions Photoelectric Effect and Wave-Particle Duality exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in HC Verma Solutions are essential questions that can be asked in the final exam. Maximum CBSE, Karnataka Board PUC Concepts of Physics Vol. 2 [English] Class 11 and 12 students prefer HC Verma Textbook Solutions to score more in exams.
Get the free view of Chapter 20, Photoelectric Effect and Wave-Particle Duality Concepts of Physics Vol. 2 [English] Class 11 and 12 additional questions for Mathematics Concepts of Physics Vol. 2 [English] Class 11 and 12 CBSE, Karnataka Board PUC, and you can use Shaalaa.com to keep it handy for your exam preparation.
