Advertisements
Chapters
2: Quantum Mechanical Model of Atom
3: Periodic Classification Of Elements
4: Hydrogen
5: Alkali and Alkaline Earth Metals
6: Gaseous State
7: Thermodynamics
8: Physical and Chemical Equilibrium
9: Solutions
10: Chemical bonding
11: Fundamentals of Organic Chemistry
▶ 12: Basic concept of organic reactions
13: Hydrocarbons
14: Haloalkanes and Haloarenes
15: Environmental Chemistry
![Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 12 - Basic concept of organic reactions Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 12 - Basic concept of organic reactions - Shaalaa.com](/images/chemistry-volume-1-and-2-english-class-11-tn-board_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 12: Basic concept of organic reactions
Below listed, you can find solutions for Chapter 12 of Tamil Nadu Board of Secondary Education Samacheer Kalvi for Chemistry - Volume 1 and 2 [English] Class 11 TN Board.
Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board 12 Basic concept of organic reactions Evaluation [Pages 175 - 176]
Choose the best answer
For the following reactions
- \[\ce{CH3CH2CH2Br + KOH → CH3 – CH = CH2 + KBr + H2O}\]
- \[\ce{(CH3)3CBr + KOH → (CH3)3COH + KBr}\]
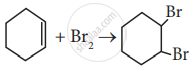
Which of the following statement is correct?
(A) is elimination, (B) and (C) are substitution
(A) is substitution, (B) and (C) are elimination
(A) and (B) are elimination and (C) is addition reaction
(A) is elimination, B is substitution and (C) is addition reaction.
What is the hybridisation state of benzyl carbonium ion?
sp2
spd2
sp3
sp2d
Decreasing order of nucleophilicity is ______.
OH– > NH2– > -OCH3 > RNH2
NH2– > OH– > -OCH3 > RNH2
NH2– > CH3O– > OH– > RNH2
CH3O– > NH2– > OH– > RNH2
Which of the following species is not electrophilic in nature?
Cl+
BH3
H3O+
+NO2
Homolytic fission of covalent bond leads to the formation of ______.
electrophile
nucleophile
Carbo cation
free radical
Hyper Conjugation is also known as
no bond resonance
Baker – nathan effect
both no bond resonance and Baker – nathan effect
none of these
Which of the group has highest + I effect?
CH3–
CH3 – CH2 –
(CH3)2 – CH-
(CH3)3 – C –
Which of the following species does not exert a resonance effect?
C6H5OH
C6H5Cl
C6H5NH2
C6H5NH3
- I effect is shown by ______.
– Cl
– Br
both – Cl and – Br
– CH3
Which of the following carbocation will be most stable?
Ph3+C –
CH3 – +CH2 –
(CH3)2 – +CH
CH2 = CH – +CH2
Assertion: Tertiary Carbocations are generally formed more easily than primary Carbocations ions.
Reason: Hyper conjucation as well as inductive effect due to additional alkyl group stabilize tertiary carbonium ions.
both assertion and reason are true and reason is the correct explanation of assertion.
both assertion and reason are true but reason is not the correct explanation of assertion.
assertion is true but reason is false.
both assertion and reason are false.
Heterolytic fission of C–C bond results in the formation of ______.
free radical
Carbanion
Carbocation
Carbanion and Carbocation
Which of the following represent a set of nucleophiles?
BF3, H2O, NH2-
AlCl3, BF3, NH3
CN, RCH2–, ROH
H+, RNH3+, :CCl2
Which of the following species does not acts as a nucleophile?
ROH
ROR
PCl3
BF3
The geometrical shape of carbocation is ______.
Linear
tetrahedral
Planar
Pyramidal
Write a short note on resonance.
Write a short note on hyperconjucation.
What is electrophiles? Give a suitable example.
What is nucleophiles? Give a suitable example.
Show the heterolysis of a covalent bond by using curved arrow notation and complete the following equation. Identify the nucelophile.
CH3 – Br + KOH →
Show the heterolysis of a covalent bond by using curved arrow notation and complete the following equation. Identify the nucelophile.
\[\ce{CH3 – O – CH3 + HI →}\]
Explain inductive effect with suitable example.
Explain the electromeric effect.
Give example for the following type of organic reaction.
β - elimination
Give example for the following type of organic reaction.
Electrophilic substitution
Solutions for 12: Basic concept of organic reactions
![Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 12 - Basic concept of organic reactions Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 12 - Basic concept of organic reactions - Shaalaa.com](/images/chemistry-volume-1-and-2-english-class-11-tn-board_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 12 - Basic concept of organic reactions
Shaalaa.com has the Tamil Nadu Board of Secondary Education Mathematics Chemistry - Volume 1 and 2 [English] Class 11 TN Board Tamil Nadu Board of Secondary Education solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Samacheer Kalvi solutions for Mathematics Chemistry - Volume 1 and 2 [English] Class 11 TN Board Tamil Nadu Board of Secondary Education 12 (Basic concept of organic reactions) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Samacheer Kalvi textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 12 Basic concept of organic reactions are Basic Concept of Organic Reactions, Different Types of Organic Reactions.
Using Samacheer Kalvi Chemistry - Volume 1 and 2 [English] Class 11 TN Board solutions Basic concept of organic reactions exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Samacheer Kalvi Solutions are essential questions that can be asked in the final exam. Maximum Tamil Nadu Board of Secondary Education Chemistry - Volume 1 and 2 [English] Class 11 TN Board students prefer Samacheer Kalvi Textbook Solutions to score more in exams.
Get the free view of Chapter 12, Basic concept of organic reactions Chemistry - Volume 1 and 2 [English] Class 11 TN Board additional questions for Mathematics Chemistry - Volume 1 and 2 [English] Class 11 TN Board Tamil Nadu Board of Secondary Education, and you can use Shaalaa.com to keep it handy for your exam preparation.
