Advertisements
Online Mock Tests
Chapters
2: Quantum Mechanical Model of Atom
3: Periodic Classification Of Elements
4: Hydrogen
5: Alkali and Alkaline Earth Metals
6: Gaseous State
7: Thermodynamics
8: Physical and Chemical Equilibrium
9: Solutions
10: Chemical bonding
11: Fundamentals of Organic Chemistry
12: Basic concept of organic reactions
13: Hydrocarbons
▶ 14: Haloalkanes and Haloarenes
15: Environmental Chemistry
![Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 14 - Haloalkanes and Haloarenes Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 14 - Haloalkanes and Haloarenes - Shaalaa.com](/images/chemistry-volume-1-and-2-english-class-11-tn-board_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 14: Haloalkanes and Haloarenes
Below listed, you can find solutions for Chapter 14 of Tamil Nadu Board of Secondary Education Samacheer Kalvi for Chemistry - Volume 1 and 2 [English] Class 11 TN Board.
Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board 14 Haloalkanes and Haloarenes Evaluation [Pages 251 - 256]
Choose the best answer
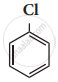
The IUPAC name of ![]() is
is
2 – Bromo pent – 3 – ene
4 – Bromo pent – 2 – ene
2 – Bromo pent – 4 – ene
4 – Bromo pent – 1 – ene
Of the following compounds, which has the highest boiling point?
n – Butyl chloride
Isobutyl chloride
t – Butyl chloride
n – Propyl chloride
Arrange the following compounds in increasing order of their density
- CCl4
- CHCl3
- CH2Cl2
- CH3Cl
D < C < B < A
C > B > A > D
A < B < C < D
C > A > B > D
With respect to the position of – Cl in the compound CH3 – CH = CH – CH2 – Cl, it is classified as ______.
Vinyl
Allyl
Secondary
Aralkyl
What should be the correct IUPAC name of diethyl chloromethane?
3 – Chloro pentane
1 – Chloropentane
1 – Chloro – 1, 1 – diethyl methane
1- Chloro-1-ethyl propane
C -X bond is strongest in ______.
Chloromethane
Iodomethane
Bromomethane
Fluoromethane
In the reaction  . X is _______.
. X is _______.
Which of the following compounds will give racemic mixture on nucleophilic substitution by OH- ion?
- \[\begin{array}{cc}\ce{CH3 - CH - CH2Br}\\
|\phantom{....}\\\ce{C2H5}\end{array}\] - \[\begin{array}{cc}\ce{CH3}\\
|\phantom{.}\\\ce{H3C - C - C2H5}\\
|\phantom{.}\\\ce{Br}\end{array}\] - \[\begin{array}{cc}\ce{H}\\
|\phantom{.}\\\ce{CH3 - C - C2H5}\\
|\phantom{.}\\\ce{Cl}\end{array}\]
(i)
(ii) and (iii)
(iii)
(i) and (ii)
The treatment of ethyl formate with excess of RMgX gives
\[\begin{array}{cc}\ce{R - C - R}\\||\\\ce{O}\end{array}\]
\[\begin{array}{cc}\ce{R - CH - R}\\|\\\phantom{..}\ce{OH}\end{array}\]
R- CHO
R- O – R
Benzene reacts with Cl2 in the presence of FeCl3 and in absence of sunlight to form ______.
Chlorobenzene
Benzyl chloride
Benzal chloride
Benzene hexachloride
The name of C2F4C12 is ______.
Freon – 112
Freon – 113
Freon – 114
Freon – 115
Which of the following reagent is helpful to differentiate ethylene dichloride and ethylidene chloride?
Zn/methanol
KOH/ethanol
aqueous KOH
ZnCl2/Con HCl
Match the compounds given in Column I with suitable items given in Column II.
| Column I (Compound) | Column II (Uses) |
||
| A | Iodoform | 1 | Fire extinguisher |
| B | Carbon tetra chloride | 2 | Insecticide |
| C | CFC | 3 | Antiseptic |
| D | DDT | 4 | Refrigerants |
A → 2, B → 4, C →1, D →3
A → 3, B → 2, C →4, D →1
A → 1, B → 2, C → 3, D → 4,
A → 3 B → 1 C → 4 D → 2
Assertion: In mono haloarenes, electrophilic substitution occurs at ortho and para positions.
Reason: Halogen atom is a ring deactivator.
If both assertion and reason are true and reason is the correct explanation of assertion.
If both assertion and reason are true but reason is not the correct explanation of assertion.
If assertion is true but reason is false.
If both assertion and reason are false.
Consider the reaction,
\[\ce{CH3CH2CH2Br + NaCN → CH3CH2CH2CN + NaBr}\]
This reaction will be the fastest in
ethanol
b) methanol
DMF (N, N’ – dimethyl formamide)
water
Freon-12 is manufactured from tetrachloro methane by ______.
Wurtz reaction
Swarts reaction
Haloform reaction
Gattermann reaction
The most easily hydrolysed molecule under SN1 condition is ______.
allyl chloride
ethyl chloride
ispropylchloride
benzyl chloride
The carbocation formed in SN1 reaction of alkyl halide in the slow step is ______.
sp3 hybridized
sp2 hybridized
sp hybridized
none of these
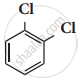
The major products obtained when chlorobenzene is nitrated with HNO3 and con H2SO4
1 – chloro – 4 – nitrobenzene
1 – chloro – 2 – nitrobenzene
1 – chloro – 3 – nitrobenzene
1 – chloro – 1 – nitrobenzene
Which one of the following is most reactive towards nucleophilic substitution reaction?
Ethylidene chloride on treatment with aqueous KOH gives ______.
acetaldehyde
ehtyleneglycol
formaldehyde
glycoxal
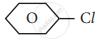
The raw material for Raschig process
chloro benzene
phenol
benzene
anisole
Chloroform reacts with nitric acid to produce ______.
nitro toluene
nitro glycerine
chloropicrin
chloropicric acid
\[\ce{acetone ->[i) CH3MgI][ii) H2O/H^{-1}]}\]X, X is ______.
2 – propanol
2 – methyl – 2 – propanol
1 – propanol
acetonol
Silver propionate when refluxed with Bromine in carbon tetrachloride gives ______.
propionic acid
chloroethane
Bromo ethane
chloro propane
Write brief answer to the following questions.
Classify the following compound in the form of alkyl, allylic, vinyl, benzylic halides.
\[\ce{CH3 – CH = CH – Cl}\]
Classify the following compound in the form of alkyl, allylic, vinyl, benzylic halides.
C6H5CH2I
Classify the following compound in the form of alkyl, allylic, vinyl, benzylic halides.
\[\begin{array}{cc}\ce{CH3 - CH - CH3}\\
|\phantom{..}\\\ce{Br}\end{array}\]
Classify the following compound in the form of alkyl, allylic, vinyl, benzylic halides.
CH2 = CH – Cl
Why chlorination of methane is not possible in dark?
How will you prepare n propyl iodide from n-propyl bromide?
Which alkyl halide from the following pair is
- chiral
- undergoes faster SN2 reaction?
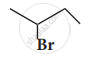
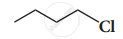
How does chlorobenzene react with sodium in the presence of ether? What is the name of the reaction?
Give reasons for the polarity of C – X bond in haloalkane.
Why is it necessary to avoid even traces of moisture during the use of Grignard reagent?
What happens when acetyl chloride is treated with an excess of CH3MgI?
Arrange the following alkyl halide in increasing order of bond enthalpy of RX.
CH3Br, CH3F, CH3Cl, CH3I
What happens when chloroform reacts with oxygen in the presence of sunlight?
Write down the possible isomers of C5H11Br and give their IUPAC and common names.
Mention any three methods of preparation of haloalkanes from alcohols.
Compare SN1 and SN2 reaction mechanisms.
Reagents and the conditions used in the reactions are given below. Complete the table by writing down the product and the name of the reaction.
| Reaction | Product | Name of the reaction |
| \[\ce{CH3CH2OH + SOCl2 ->[pyridine] ?}\] | ______ | ______ |
| \[\ce{CH3CH2Br + AgF ->}\] ? | ______ | ______ |
| \[\ce{C6H5Cl + Na ->[Ether]}\] | ______ | ______ |
Discuss the aromatic nucleophilic substitutions reaction of chlorobenzene.
Account for the following
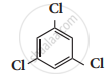
t – butyl chloride reacts with aqueous KOH by SN1 mechanism while n – butyl chloride reacts with SN2 mechanism.
p – dichlorobenzene has a higher melting point than those of o – and m – dichlorobenzene.
In an experiment ethyliodide in ether is allowed to stand over magnesium pieces. Magnesium dissolves and product is formed
- Name the product and write the equation for the reaction.
- Why all the reagents used in the reaction should be dry? Explain.
- How is acetone prepared from the product obtained in the experiment.
Write a chemical reaction useful to prepare the following:
Freon – 12 from Carbon tetrachloride
Write a chemical reaction useful to prepare the following:
Carbon tetrachloride from carbon disulphide.
What are Freons?
Discuss uses of ferons.
Discuss the environmental effects of freons.
Predict the product when Bromo ethane is treated with the following.
KNO2
Predict the products when Bromo ethane is treated with the following.
AgNO2
Explain the mechanism of SN1 reaction by highlighting the stereochemistry behind it.
Write a short note on raschig process.
Write a short note on Dows Process.
Write a short note on Darzen’s process.
Starting from CH3MgI, How will you prepare the following?
Acetic acid
Starting from CH3MgI, How will you prepare the following?
Acetone
Starting from CH3MgI, How will you prepare the following?
Ethyl acetate
Starting from CH3MgI, How will you prepare the following?
Iso propyl alcohol
Starting from CH3MgI, How will you prepare the following?
Methyl cyanide
Complete the following reaction.
\[\ce{CH3 - CH = CH2 + HBr ->[Peroxide]}\]
Complete the following reaction.
\[\ce{CH3 - CH2 - Br + NaSH ->[alcohol][H2O]}\]
Complete the following reaction.
\[\ce{C6H5Cl + Mg ->[THF]}\]
Complete the following reaction.
\[\ce{CHCl3 + HNO3 ->[\Delta]}\]
Complete the following reaction.
\[\ce{CCl4 + H2O ->[\Delta]}\]
Explain the preparation of the following compound.
DDT
Explain the preparation of the following compound.
Chloroform
Explain the preparation of the following compound.
Biphenyl
Explain the preparation of the following compound.
Chloropicrin
Explain the preparation of the following compound.
Freon-12
An organic compound (A) with molecular formula C2H5Cl reacts with KOH gives compounds (B) and with alcoholic KOH gives compound (C). Identify (A), (B), (C).
The simplest alkene (A) reacts with HCl to form a compound (B). Compound (B) reacts with ammonia to form compound (C) of molecular formula C2H7N. Compound (C) undergoes carbylamine test. Identify (A),
(B) and (C).
A hydrocarbon C3H6(A) reacts with HBr to form compound (B). Compound (B) reacts with aqueous potassium hydroxide to give (C) of molecular formula C3H6O. What are the (A), (B) and (C). Explain the reactions.
Two isomers (A) and (B) have the same molecular formula C2H4Cl2. Compound (A) reacts with aqueous KOH gives compound (C) of molecular formula C2H4O. Compound (B) reacts with aqueous KOH gives compound (D) of molecular formula C2H6O2. Identify (A), (B), (C) and (D).
Solutions for 14: Haloalkanes and Haloarenes
![Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 14 - Haloalkanes and Haloarenes Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 14 - Haloalkanes and Haloarenes - Shaalaa.com](/images/chemistry-volume-1-and-2-english-class-11-tn-board_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 14 - Haloalkanes and Haloarenes
Shaalaa.com has the Tamil Nadu Board of Secondary Education Mathematics Chemistry - Volume 1 and 2 [English] Class 11 TN Board Tamil Nadu Board of Secondary Education solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Samacheer Kalvi solutions for Mathematics Chemistry - Volume 1 and 2 [English] Class 11 TN Board Tamil Nadu Board of Secondary Education 14 (Haloalkanes and Haloarenes) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Samacheer Kalvi textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry - Volume 1 and 2 [English] Class 11 TN Board chapter 14 Haloalkanes and Haloarenes are Introduction to Haloalkanes and Haloarenes, Classification of Organic Halogen Compounds, Haloalkanes, Organo Metallic Compounds, Haloarenes, Poly Halogen Compounds.
Using Samacheer Kalvi Chemistry - Volume 1 and 2 [English] Class 11 TN Board solutions Haloalkanes and Haloarenes exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Samacheer Kalvi Solutions are essential questions that can be asked in the final exam. Maximum Tamil Nadu Board of Secondary Education Chemistry - Volume 1 and 2 [English] Class 11 TN Board students prefer Samacheer Kalvi Textbook Solutions to score more in exams.
Get the free view of Chapter 14, Haloalkanes and Haloarenes Chemistry - Volume 1 and 2 [English] Class 11 TN Board additional questions for Mathematics Chemistry - Volume 1 and 2 [English] Class 11 TN Board Tamil Nadu Board of Secondary Education, and you can use Shaalaa.com to keep it handy for your exam preparation.