Advertisements
Advertisements
Questions
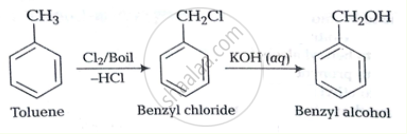
How will you bring about the following conversion?
Toluene to benzyl alcohol
How the following conversion can be carried out?
Toluene to benzyl alcohol
Solution
APPEARS IN
RELATED QUESTIONS
Discuss the mechanism of alkaline hydrolysis of bromomethane.
Out of 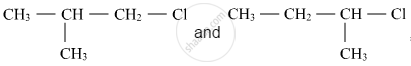 , which is more reactive towards SN1 reaction and why?
, which is more reactive towards SN1 reaction and why?
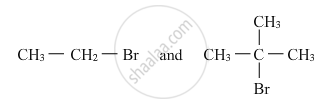
Which would undergo SN2 reaction faster in the following pair and why ?
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2CH2OH + SOCl2 ->}\]
The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
How the following conversion can be carried out?
Ethyl chloride to propanoic acid
What is the action of the following on ethyl bromide:
silver acetate
Which of the following is optically inactive?
Which of the following is an example of SN2 reaction?
Which among MeX, RCH2X, R2CHX and R3CX is most reactive towards SN2 reaction?
Racemic compound has ____________.
2-Bromopentane is heated with potassium ethoxide in ethanol. The major product obtained is ____________.
The increasing order of nucleophilicity would be:
A primary alkyl halide would prefer to undergo ______.
Which of the compounds will react faster in SN1 reaction with the –OH ion?
\[\ce{CH3-CH2-Cl}\] or \[\ce{C6H5-CH2-Cl}\]
Write the structures and names of the compounds formed when compound ‘A’ with molecular formula, \[\ce{C7H8}\] is treated with \[\ce{Cl2}\] in the presence of \[\ce{FeCl3}\].
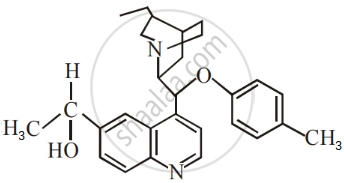
The number of chiral carbons present in the molecule given below is ______.
Give the mechanism of the following reaction:
\[\ce{CH3CH2OH ->[H2SO4][413 K] CH3CH2-O-CH2CH3 + H2O}\]
An organic compound A with the molecular formula (+) C4H9Br undergoes hydrolysis to form (+) C4H9OH. Give the structure of A and write the mechanism of the reaction.
Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
\[\begin{array}{cc}\ce{CH3CHCH2CH2Br}\\|\phantom{.........}\\\ce{CH3}\phantom{......}\end{array}\] or \[\begin{array}{cc}\ce{CH3CH2CHCH2Br}\\\phantom{}|\\\phantom{...}\ce{CH3}\end{array}\]
