Advertisements
Advertisements
Question
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH2CH2OH + SOCl2 ->}\]
Solution
\[\ce{\underset{Propan-1-ol}{CH3CH2CH2OH} + SOCl2 ->[nucleophilic substitution] \underset{1-Chloropropane}{CH3CH2CH2Cl} + HCl\uparrow + SO2\uparrow}\]
APPEARS IN
RELATED QUESTIONS
Write the structures of A, B and C in the following:

Which alkyl halide from the following pair would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
CH3CH2CH2CH2Br or \[\begin{array}{cc}
\ce{CH3CH2CHCH3}\\
\phantom{...}|\\
\phantom{....}\ce{Br}\
\end{array}\]
Write the structure of the major organic product in the following reaction:
\[\ce{CH3CH(Br)CH2CH3 + NaOH ->[water]}\]
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
2-Bromo-2-methylbutane, 1-Bromopentane, 2-Bromopentane
Arrange the compounds of the following set in order of reactivity towards SN2 displacement:
1-Bromobutane, 1-Bromo-2, 2-dimethylpropane, 1-Bromo-2-methylbutane, 1-Bromo-3-methylbutane
Which one of the following halogen compounds is difficult to be hydrolysed by SN1 mechanism?
Optically active isomers but not mirror images are called ____________.
The process of separation of a racemic modification into d and l-enantiomers is called ____________.
Racemic compound has ____________.
Assertion: KCN reacts with methyl chloride to give methyl isocyanide.
Reason: CN– is an ambident nucleophile.
Which reagent will you use for the following reaction?
\[\ce{CH3CH2CH2CH3 -> CH3CH2CH2CH2Cl + CH3CH2CHClCH3}\]
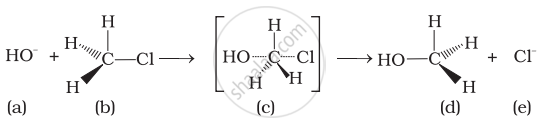
Which of the statements are correct about above reaction?
(i) (a) and (e) both are nucleophiles.
(ii) In (c) carbon atom is sp3 hybridised.
(iii) In (c) carbon atom is sp2 hybridised.
(iv) (a) and (e) both are electrophiles.
Which of the following statements are correct about the kinetics of this reaction?
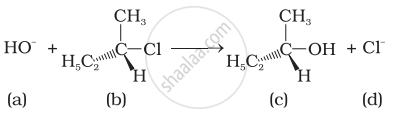
(i) The rate of reaction depends on the concentration of only (b).
(ii) The rate of reaction depends on concentration of both (a) and (b).
(iii) Molecularity of reaction is one.
(iv) Molecularity of reaction is two.
Ethylene chloride and ethylidene chloride are isomers. Identify the correct statements.
(i) Both the compounds form same product on treatment with alcoholic KOH.
(ii) Both the compounds form same product on treatment with aq.NaOH.
(iii) Both the compounds form same product on reduction.
(iv) Both the compounds are optically active.
In which reaction mechanism carbocation is formed?
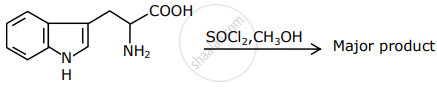
The major product formed in the following reaction is:
Which of the following compounds will show retention in configuration on nucleophile substitution by OH− ion?
An organic compound A with the molecular formula (+) C4H9Br undergoes hydrolysis to form (+) C4H9OH. Give the structure of A and write the mechanism of the reaction.
Identify the product in the following reaction: 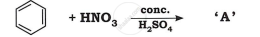
Acetic anhydride from acetic acid
