Advertisements
Online Mock Tests
Chapters
2: p-Block Elements - I
3: p-Block Elements - II
4: Transition and Inner Transition Elements
5: Coordination Chemistry
6: Solid State
▶ 7: Chemical Kinetics
8: Ionic Equilibrium
9: Electro Chemistry
10: Surface Chemistry
11: Hydroxy Compounds and Ethers
12: Carbonyl Compounds and Carboxylic Acids
13: Organic Nitrogen Compounds
14: Biomolecules
15: Chemistry in Everyday Life
![Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 12 TN Board chapter 7 - Chemical Kinetics Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 12 TN Board chapter 7 - Chemical Kinetics - Shaalaa.com](/images/chemistry-volume-1-and-2-english-class-12-tn-board_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 7: Chemical Kinetics
Below listed, you can find solutions for Chapter 7 of Tamil Nadu Board of Secondary Education Samacheer Kalvi for Chemistry - Volume 1 and 2 [English] Class 12 TN Board.
Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 12 TN Board 7 Chemical Kinetics Evaluation [Pages 226 - 231]
Choose the best answer
For a first-order reaction \[\ce{A -> B}\] the rate constant is x min−1. If the initial concentration of A is 0.01 M, the concentration of A after one hour is given by the expression.
0.01 e−x
1 × 10−2 (1 − e−60x)
(1 × 10−2) e−60x
none of these
A zero-order reaction \[\ce{X -> Product}\], with an initial concentration 0.02 M has a half-life of 10 min. if one starts with concentration 0.04 M, then the half-life is
10 s
5 min
20 min
cannot be predicted using the given information
Among the following graphs showing the variation of rate constant with temperature (T) for a reaction, the one that exhibits Arrhenius behavior over the entire temperature range is
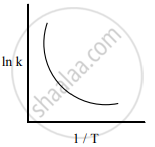
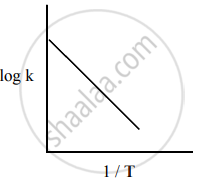
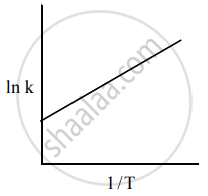
Both
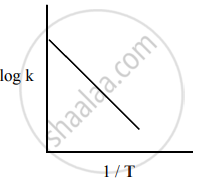
and

For a first order reaction \[\ce{A ->Product}\] with initial concentration x mol L−1, has a half life period of 2.5 hours. For the same reaction with initial concentration `("x"/2)` mol L−1 the half life is
(2.5 × 2) hours
`(2.5/2)` hours
2.5 hours
Without knowing the rate constant, t1/2 cannot be determined from the given data
For the reaction, \[\ce{2NH3 -> N2 + 3H2}\], if `(-"d" ["NH"_3])/"dt"` = k1 [NH3], `("d" ["N"_2])/"dt"` = k2 [NH3], `("d" ["H"_2])/"dt"` = k3 [NH3] then the relation between k1, k2 and k3 is
k1 = k2 = k3
k1 = 3 k2 = 2 k3
1.5 k1 = 3 k2 = k3
2 k1 = k2 = 3 k3
The decomposition of phosphine (PH3) on tungsten at low pressure is a first-order reaction. It is because the
rate is proportional to the surface coverage
rate is inversely proportional to the surface coverage
rate is independent of the surface coverage
rate of decomposition is slow
For a reaction Rate = `"k" ["acetone"]^(3/2)` then unit of rate constant and rate of reaction respectively is
`("mol L"^-1 "s"^-1)`, `("mol"^((-1)/2) "L"^(1/2) "s"^-1)`
`("mol"^((-1)/2) "L"^(1/2) "s"^-1)`, `("mol L"^-1 "s"^-1)`
`("mol"^(1/2) "L"^(1/2) "s"^-1)`, `("mol L"^-1 "s"^-1)`
`("mol L s"^-1)`, `("mol"^(1/2) "L"^(1/2) "s")`
The addition of a catalyst during a chemical reaction alters which of the following quantities?
Enthalpy
Activation energy
Entropy
Internal energy
Consider the following statements:
(i) increase in concentration of the reactant increases the rate of a zero-order reaction.
(ii) rate constant k is equal to collision frequency A if Ea = 0
(iii) rate constant k is equal to collision frequency A if Ea = ∞
(iv) a plot of ln (k) vs T is a straight line.
(v) a plot of ln (k) vs `(1/"T")` is a straight line with a positive slope.
Correct statements are
(ii) only
(ii) and (iv)
(ii) and (v)
(i), (ii) and (v)
In a reversible reaction, the enthalpy change and the activation energy in the forward direction are respectively −x kJ mol−1 and y kJ mol−1. Therefore , the energy of activation in the backward direction is
(y − x) kJ mol−1
(x + y) J mol−1
(x − y) kJ mol−1
(x + y) × 103 J mol−1
What is the activation energy for a reaction if its rate doubles when the temperature is raised from 200 K to 400 K? (R = 8.314 JK−1mol−1)
234.65 kJ mol−1
434.65 kJ mol−1
2.305 kJ mol−1
334.65 J mol−1

This reaction follows first-order kinetics. The rate constant at particular temperature is 2.303 × 10−2 hour−1. The initial concentration of cyclopropane is 0.25 M. What will be the concentration of cyclopropane after 1806 minutes? (log 2 = 0.3010)
0.125 M
0.215 M
0.25 × 2.303 M
0.05 M
For a first-order reaction, the rate constant is 6.909 min−1 the time taken for 75% conversion in minutes is
`(3/2) log 2`
`(2/3) log 2`
`(3/2) log (3/4)`
`(2/3) log (4/3)`
In a first order reaction \[\ce{x -> y}\]; if k is the rate constant and the initial concentration of the reactant x is 0.1 M, then, the half life is
`((log 2)/"k")`
`(0.693/((0.1) "k"))`
`((ln 2)/"k")`
none of these
Predict the rate law of the following reaction based on the data given below.
\[\ce{2A + B -> C + 3D}\]
| Reaction number | [A] (min) |
[B] (min) |
Initial rate (M s−1) |
| 1 | 0.1 | 0.1 | x |
| 2 | 0.2 | 0.1 | 2x |
| 3 | 0.1 | 0.2 | 4x |
| 4 | 0.2 | 0.2 | 8x |
rate = k [A]2 [B]
rate = k [A] [B]2
rate = k [A] [B]
rate = `"k" ["A"]^(1/2) ["B"]^(3/2)`
Assertion: rate of reaction doubles when the concentration of the reactant is doubles if it is a first-order reaction.
Reason: rate constant also doubles.
Both assertion and reason are true and reason is the correct explanation of assertion.
Both assertion and reason are true but reason is not the correct explanation of assertion.
Assertion is true but reason is false.
Both assertion and reason are false.
The rate constant of a reaction is 5.8 × 10−2 s−1. The order of the reaction is ____________.
First order
Zero order
Second order
Third order
For the reaction \[\ce{N2O5_{(g)} -> 2NO2_{(g)} + 1/2O2_{(g)}}\], the value of rate of disappearance of N2O5 is given as 6.5 × 10−2 mol L−1s−1. The rate of formation of NO2 and O2 is given respectively as
(3.25 × 10−2 mol L-1s−1) and (1.3 × 10−2 mol L−1s−1)
(1.3 × 10−2 mol L−1s−1) and (3.25 × 10−2 mol L−1s−1)
(1.3 × 10−1 mol L−1s−1) and (3.25 × 10−2 mol L−1s−1)
None of these
During the decomposition of H2O2 to give dioxygen, 48 g O2 is formed per minute at certain point of time. The rate of formation of water at this point is
0.75 mol min−1
1.5 mol min−1
2.25 mol min−1
3.0 mol min−1
If the initial concentration of the reactant is doubled, the time for half reaction is also doubled. Then the order of the reaction is ____________.
Zero
One
Fraction
None
In a homogeneous reaction \[\ce{A -> B + C + D}\], the initial pressure was P0 and after time t it was P. Expression for rate constant in terms of P0, P and t will be
`"k" = (2.303/"t") log ((2"P"_0)/(3"P"_0 - "P"))`
`"k" = (2.303/"t") log ((2"P"_0)/("P"_0 - "P"))`
`"k" = (2.303/"t") log ((3"P"_0 - "P")/(2"P"_0))`
`"k" = (2.303/"t") log ((2"P"_0)/(3"P"_0 - 2"P"))`
If 75% of a first order reaction was completed in 60 minutes, 50% of the same reaction under the same conditions would be completed in ____________.
20 minutes
30 minutes
35 minutes
75 minutes
The half life period of a radioactive element is 140 days. After 560 days, 1 g of element will be reduced to
`(1/2) "g"`
`(1/4) "g"`
`(1/8) "g"`
`(1/16) "g"`
The correct difference between first and second order reactions is that
A first order reaction can be catalysed; a second order reaction cannot be catalysed.
The half life of a first order reaction does not depend on [A0]; the half life of a second order reaction does depend on [A0].
The rate of a first order reaction does not depend on reactant concentrations; the rate of a second order reaction does depend on reactant concentrations.
The rate of a first order reaction does depend on reactant concentrations; the rate of a second order reaction does not depend on reactant concentrations.
After 2 hours, a radioactive substance becomes `(1/16)^"th"` of original amount. Then the half life ( in min) is
60 minutes
120 minutes
30 minutes
15 minutes
Answer the following questions:
Define average rate.
Define instantaneous rate.
Define rate law.
Define rate constant.
Derive integrated rate law for a zero-order reaction \[\ce{A -> Product}\].
Define half life of a reaction.
Show that for a first order reaction half life is independent of initial concentration.
What is an elementary reaction?
Give the differences between order and molecularity of a reaction.
Explain the rate determining step with an example.
Describe the graphical representation of first order reaction.
Write the rate law for the following reaction.
A reaction that is `3/2` order in x and zero order in y.
Write the rate law for the following reaction.
A reaction that is second order in NO and first order in Br2.
Explain the effect of catalyst on reaction rate with an example.
The rate law for a reaction of A, B and C has been found to be rate = `"k" ["A"]^2["B"] ["L"]^(3/2)`. How would the rate of reaction change when
- Concentration of [L] is quadrupled
- Concentration of both [A] and [B] are doubled
- Concentration of [A] is halved
- Concentration of [A] is reduced to `(1/3)` and concentration of [L] is quadrupled.
The rate of formation of a dimer in a second order reaction is 7.5 × 10−3 mol L−1s−1 at 0.05 mol L−1 monomer concentration. Calculate the rate constant.
For a reaction \[\ce{x + y + z -> products}\] the rate law is given by rate = `"k" ["x"]^(3/2) ["y"]^(1/2)` what is the overall order of the reaction and what is the order of the reaction with respect to z.
Explain briefly the collision theory of bimolecular reactions.
Write Arrhenius equation and explains the terms involved.
The decomposition of Cl2O7 at 500 K in the gas phase to Cl2 and O2 is a first order reaction. After 1 minute at 500 K, the pressure of Cl2O7 falls from 0.08 to 0.04 atm. Calculate the rate constant in s−1.
Give two examples for zero order reaction.
Explain pseudo-first-order reaction with an example.
Identify the order for the following reaction.
Rusting of Iron
Identify the order for the following reaction.
Radioactive disintegration of 92U238
Identify the order for the following reaction.
\[\ce{2A + 3B -> products}\]; rate = `"k" ["A"]^(1/2) ["B"]^2`
A gas phase reaction has energy of activation 200 kJ mol−1. If the frequency factor of the reaction is 1.6 × 1013 s−1. Calculate the rate constant at 600 K. `("e"^-40.09 = 3.8 xx 10^-18)`
For the reaction \[\ce{2x + y -> L}\] find the rate law from the following data.
| [x] (M) |
[y] (M) |
rate (M s−1) |
| 0.2 | 0.02 | 0.15 |
| 0.4 | 0.02 | 0.30 |
| 0.4 | 0.08 | 1.20 |
How do concentrations of the reactant influence the rate of reaction?
How do nature of the reactant influence rate of reaction?
The rate constant for a first order reaction is 1.54 × 10−3 s−1. Calculate its half life time.
The half life of the homogeneous gaseous reaction \[\ce{SO2Cl2 -> SO2 + Cl2}\] which obeys first order kinetics is 8.0 minutes. How long will it take for the concentration of SO2Cl2 to be reduced to 1% of the initial value?
The time for half change in a first order decomposition of a substance A is 60 seconds. Calculate the rate constant. How much of A will be left after 180 seconds?
A zero order reaction is 20% complete in 20 minutes. Calculate the value of the rate constant. In what time will the reaction be 80% complete?
The activation energy of a reaction is 22.5 k Cal mol−1 and the value of rate constant at 40°C is 1.8 × 10−5 s−1. Calculate the frequency factor, A.
Benzene diazonium chloride in aqueous solution decomposes according to the equation \[\ce{C6H5N2Cl -> C6H5Cl + N2}\]. Starting with an initial concentration of 10 g L−1, the volume of N2 gas obtained at 50°C at different intervals of time was found to be as under:
| t (min): | 6 | 12 | 18 | 24 | 30 | ∞ |
| Vol. of N2 (ml) |
19.3 | 32.6 | 41.3 | 46.5 | 50.4 | 58.3 |
Show that the above reaction follows the first order kinetics. What is the value of the rate constant?
From the following data, show that the decomposition of hydrogen peroxide is a reaction of the first order:
| t (min) | 0 | 10 | 20 |
| V (ml) | 46.1 | 29.8 | 19.3 |
Where t is the time in minutes and V is the volume of standard KMnO4 solution required for titrating the same volume of the reaction mixture.
A first order reaction is 40% complete in 50 minutes. Calculate the value of the rate constant. In what time will the reaction be 80% complete?
Solutions for 7: Chemical Kinetics
![Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 12 TN Board chapter 7 - Chemical Kinetics Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 12 TN Board chapter 7 - Chemical Kinetics - Shaalaa.com](/images/chemistry-volume-1-and-2-english-class-12-tn-board_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Samacheer Kalvi solutions for Chemistry - Volume 1 and 2 [English] Class 12 TN Board chapter 7 - Chemical Kinetics
Shaalaa.com has the Tamil Nadu Board of Secondary Education Mathematics Chemistry - Volume 1 and 2 [English] Class 12 TN Board Tamil Nadu Board of Secondary Education solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Samacheer Kalvi solutions for Mathematics Chemistry - Volume 1 and 2 [English] Class 12 TN Board Tamil Nadu Board of Secondary Education 7 (Chemical Kinetics) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Samacheer Kalvi textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Chemistry - Volume 1 and 2 [English] Class 12 TN Board chapter 7 Chemical Kinetics are Integrated Rate Equations, Rate of Chemical Reaction, First Order Reactions, Zero Order Reactions, Molecularity, Collision Theory, Arrhenius Equation – the Effect of Temperature on Reaction Rate, Factors Affecting the Reaction Rate, Pseudo First Order Reaction, Half Life Period of a Reaction.
Using Samacheer Kalvi Chemistry - Volume 1 and 2 [English] Class 12 TN Board solutions Chemical Kinetics exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Samacheer Kalvi Solutions are essential questions that can be asked in the final exam. Maximum Tamil Nadu Board of Secondary Education Chemistry - Volume 1 and 2 [English] Class 12 TN Board students prefer Samacheer Kalvi Textbook Solutions to score more in exams.
Get the free view of Chapter 7, Chemical Kinetics Chemistry - Volume 1 and 2 [English] Class 12 TN Board additional questions for Mathematics Chemistry - Volume 1 and 2 [English] Class 12 TN Board Tamil Nadu Board of Secondary Education, and you can use Shaalaa.com to keep it handy for your exam preparation.
