Advertisements
Chapters
![Samacheer Kalvi solutions for Physics - Volume 1 and 2 [English] Class 11 TN Board chapter 9 - Kinetic Theory of Gases Samacheer Kalvi solutions for Physics - Volume 1 and 2 [English] Class 11 TN Board chapter 9 - Kinetic Theory of Gases - Shaalaa.com](/images/physics-volume-1-and-2-english-class-11-tn-board_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Advertisements
Solutions for Chapter 9: Kinetic Theory of Gases
Below listed, you can find solutions for Chapter 9 of Tamil Nadu Board of Secondary Education Samacheer Kalvi for Physics - Volume 1 and 2 [English] Class 11 TN Board.
Samacheer Kalvi solutions for Physics - Volume 1 and 2 [English] Class 11 TN Board 9 Kinetic Theory of Gases Evaluation [Pages 183 - 186]
Multiple choice questions
A particle of mass m is moving with speed u in a direction which makes 60° with respect to the x-axis. It undergoes elastic collision with the wall. What is the change in momentum in x and y direction?
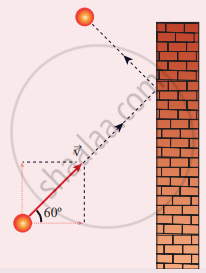
∆px = −mu, ∆py = 0
∆px = – 2mu, ∆py = 0
∆px = 0, ∆py = mu
∆px = mu, ∆py = 0
A sample of an ideal gas is at equilibrium. Which of the following quantity is zero?
rms speed
average speed
average velocity
most probable speed
An ideal gas is maintained at constant pressure. If the temperature of an ideal gas increases from 100 K to 10000 K then the rms speed of the gas molecules
increases by 5 times
increases by 10 times
remains same
increases by 7 times
Two identically sized rooms A and B are connected by an open door. If the room A is air-conditioned such that its temperature is 4°C lesser than room B, which room has more air in it?
Room A
Room B
Both room has same air
Cannot be determined
The average translational kinetic energy of gas molecules depends on ____________.
number of moles and T
only on T
P and T
P only
If the internal energy of an ideal gas U and volume V are doubled then the pressure ____________.
doubles
remains same
halves
quadruples
The ratio γ = `"C"_"p"/"C"_"v"` for a gas mixture consisting of 8 g of helium and 16 g of oxygen is ____________.
`23/15`
`15/23`
`27/17`
`17/27`
A container has one mole of the monoatomic ideal gas. Each molecule has f degrees of freedom. What is the ratio of γ = `"C"_"p"/"C"_"v"`?
f
`"f"/2`
`"f"/("f" + 2)`
`("f" + 2)/"f"`
If the temperature and pressure of a gas is doubled the mean free path of the gas molecules ____________.
remains same
doubled
tripled
quadrapoled
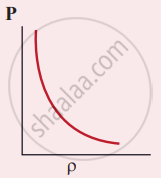
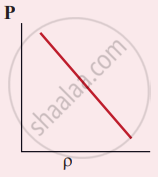
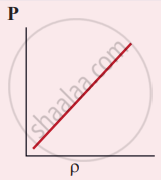
Which of the following shows the correct relationship between the pressure and density of an ideal gas at constant temperature?
A sample of gas consists of μ1 moles of monoatomic molecules, μ2 moles of diatomic molecules and μ3 moles of linear triatomic molecules. The gas is kept at a high temperature. What is the total number of degrees of freedom?
[3μ1 + 7(μ2 + μ3)] NA
[3μ1 + 7μ2 + 6μ3] NA
[7μ1 + 3(μ2 + μ3)] NA
[3μ1 + 6(μ2 + μ3)] NA
If sP and sV denote the specific heats of nitrogen gas per unit mass at constant pressure and constant volume respectively, then
SP – SV = 28 R
SP – SV = `"R"/28`
SP – SV = `"R"/14`
SP – SV = R
Which of the following gases will have the least rms speed at a given temperature?
Hydrogen
Nitrogen
Oxygen
Carbon dioxide
For a given gas molecule at a fixed temperature, the area under the Maxwell-Boltzmann distribution curve is equal to ____________.
`"PV"/"kT"`
`"kT"/"PV"`
`"P"/"NkT"`
PV
The following graph represents the pressure versus number density for an ideal gas at two different temperatures T1 and T2. The graph implies
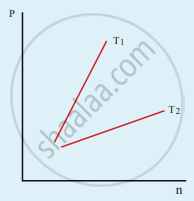
T1 = T2
T1 > T2
T1 < T2
Cannot be determined
Short answer questions
What is the microscopic origin of pressure?
What is the microscopic origin of temperature?
Why moon has no atmosphere?
Write the expression for rms speed, average speed and most probable speed of a gas molecule.
What is the relation between the average kinetic energy and pressure?
Define the term degrees of freedom.
State the law of equipartition of energy.
Define mean free path and write down its expression.
Deduce Charles’ law based on kinetic theory.
Deduce Boyle’s law based on kinetic theory.
Deduce Avogadro’s law based on kinetic theory.
List the factors affecting the mean free path.
What is the reason for the Brownian motion?
Long answer questions
Write down the postulates of the kinetic theory of gases.
Derive an expression for the pressure exerted by a gas on the basis of the kinetic theory of gases.
Explain in detail the kinetic interpretation of temperature.
Describe the total degrees of freedom for monoatomic molecule, diatomic molecule and triatomic molecule.
Derive the ratio of two specific heat capacities of monoatomic, diatomic and triatomic molecules.
Explain in detail the Maxwell Boltzmann distribution function.
Derive the expression for the mean free path of the gas.
Describe the Brownian motion.
Numerical Problems
A fresh air is composed of nitrogen N2 (78%) and oxygen O2 (21%). Find the rms speed of N2 and O2 at 20°C.
If the rms speed of methane gas in the Jupiter’s atmosphere is 471.8 ms−1, shows that the surface temperature of Jupiter is sub-zero.
Calculate the temperature at which the rms velocity of a gas triples its value at S.T.P. (standard temperature T1 = 273 K)
A gas is at temperature 80°C and pressure 5 × 10−10 Nm−2. What is the number of molecules per m3 if Boltzmann’s constant is 1.38 × 10−23 J K−1
If 1020 oxygen molecules per second strike 4 cm2 of wall at an angle of 30° with the normal when moving at a speed of 2 × 103 ms−1, find the pressure exerted on the wall. (mass of one oxygen atom = 2.67 × 10−26 kg)
During an adiabatic process, the pressure of a mixture of monatomic and diatomic gases is found to be proportional to the cube of the temperature. Find the value of γ = `("C"_"P"/"C"_"V")`
Calculate the mean free path of air molecules at STP. The diameter of N2 and O2 is about 3 × 10−10 m
A gas made of a mixture of 2 moles of oxygen and 4 moles of argon at temperature T. Calculate the energy of the gas in terms of RT. Neglect the vibrational modes.
Estimate the total number of air molecules in a room of a capacity of 25 m3 at a temperature of 27°C.
Solutions for 9: Kinetic Theory of Gases
![Samacheer Kalvi solutions for Physics - Volume 1 and 2 [English] Class 11 TN Board chapter 9 - Kinetic Theory of Gases Samacheer Kalvi solutions for Physics - Volume 1 and 2 [English] Class 11 TN Board chapter 9 - Kinetic Theory of Gases - Shaalaa.com](/images/physics-volume-1-and-2-english-class-11-tn-board_6:5f2b1b2038084cf381bfa42c826a928c.jpg)
Samacheer Kalvi solutions for Physics - Volume 1 and 2 [English] Class 11 TN Board chapter 9 - Kinetic Theory of Gases
Shaalaa.com has the Tamil Nadu Board of Secondary Education Mathematics Physics - Volume 1 and 2 [English] Class 11 TN Board Tamil Nadu Board of Secondary Education solutions in a manner that help students grasp basic concepts better and faster. The detailed, step-by-step solutions will help you understand the concepts better and clarify any confusion. Samacheer Kalvi solutions for Mathematics Physics - Volume 1 and 2 [English] Class 11 TN Board Tamil Nadu Board of Secondary Education 9 (Kinetic Theory of Gases) include all questions with answers and detailed explanations. This will clear students' doubts about questions and improve their application skills while preparing for board exams.
Further, we at Shaalaa.com provide such solutions so students can prepare for written exams. Samacheer Kalvi textbook solutions can be a core help for self-study and provide excellent self-help guidance for students.
Concepts covered in Physics - Volume 1 and 2 [English] Class 11 TN Board chapter 9 Kinetic Theory of Gases are Kinetic Theory, Degrees of Freedom, Mean (or) Average Speed, Most Probable Speed (Vmp), Maxwell-Boltzmann Speed Distribution Function, Some Elementary Deductions from Kinetic Theory of Gases, Expression for Pressure Exerted by a Gas, Interpretation of Temperature in Kinetic Theory, Relation Between Pressure and Mean Kinetic Energy, Root Mean Square (RMS) Speed, Law of Equipartiton of Energy, Mean Free Path, Brownian Motion.
Using Samacheer Kalvi Physics - Volume 1 and 2 [English] Class 11 TN Board solutions Kinetic Theory of Gases exercise by students is an easy way to prepare for the exams, as they involve solutions arranged chapter-wise and also page-wise. The questions involved in Samacheer Kalvi Solutions are essential questions that can be asked in the final exam. Maximum Tamil Nadu Board of Secondary Education Physics - Volume 1 and 2 [English] Class 11 TN Board students prefer Samacheer Kalvi Textbook Solutions to score more in exams.
Get the free view of Chapter 9, Kinetic Theory of Gases Physics - Volume 1 and 2 [English] Class 11 TN Board additional questions for Mathematics Physics - Volume 1 and 2 [English] Class 11 TN Board Tamil Nadu Board of Secondary Education, and you can use Shaalaa.com to keep it handy for your exam preparation.