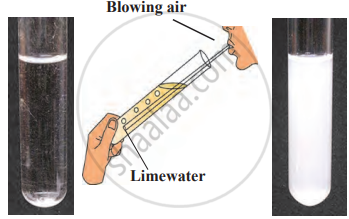
1. Aim: To study the reaction of carbon with oxygen and observe the changes in the released gas and its effect on litmus paper.
2. Requirements: coal, matchbox, moist blue litmus paper.
3. Procedure
- Ignite a piece of coal using a matchbox.
- Hold a moist blue litmus paper over the gas released during the combustion of coal.
- Observe the changes in the litmus paper and note the reaction of coal with the gas in the air.
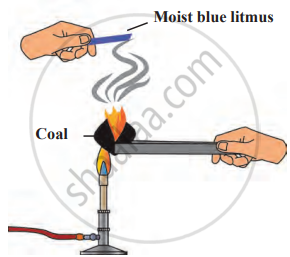
Reaction of carbon with oxygen
4. Conclusion: The coal reacts with oxygen from the air, forming carbon dioxide (CO₂). The moist blue litmus paper turns red, indicating the presence of an acidic gas (CO₂). The chemical reaction occurring is C+O₂→CO₂.This experiment demonstrates that carbon reacts with oxygen to produce carbon dioxide, which is acidic in nature.